Is Iodine More Soluble In Water Or Hexane. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. More trending news?visit yahoo home. Iodine plays an important role in the. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Glucose is very polar and dissolves in polar water. What are the health effects of iodine in water? Iodine is soluble in both ethanol and hexane. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Get more help from chegg. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. Iodine is not soluble in water because iodine is nonpolar and water is polar.
Solubility Of Iodine In A Water And Hexane Mixture Please Help The Student Room
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcsntsxe65mvf3nwj3vlkcmy8wmgonqadjd9mw Usqp Cau. Iodine is not soluble in water because iodine is nonpolar and water is polar. Iodine is soluble in both ethanol and hexane. More trending news?visit yahoo home. Get more help from chegg. Glucose is very polar and dissolves in polar water. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. What are the health effects of iodine in water? According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. Iodine plays an important role in the.
For ni(oh)2, adding h^+ reacts with oh to form h2o so yes, it.
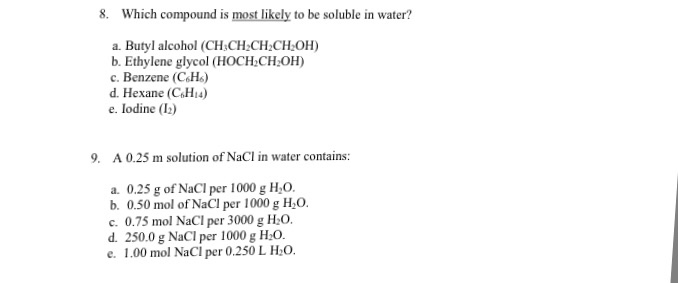
Acid, ketoses are dehydrated more rapidly to give furfural derivatives and on condensation with resorcinol give cherry red complex. Suppose that nacl is added to hexane instead of water. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Which of the following intermolecular forces will exist in the system? Toluene ethylene glycol hexane water. Determine whether each of the following solutes is more soluble in hot water? For agi the acid would be hi and that is a strong acid; If we add hexane to water, the hexane will float on the top of the water with no apparent mixing. All group ia and nh 4 + salts. Acid, ketoses are dehydrated more rapidly to give furfural derivatives and on condensation with resorcinol give cherry red complex. Iodine is made up of molecules of i2. Iodine is soluble in both ethanol and hexane. In water, 0.03 g/100 cc at 20 deg c. Solubility is a result of an interaction between polar water molecules and the ions that make up a crystal. Solubility rules for soluble substances. For ni(oh)2, adding h^+ reacts with oh to form h2o so yes, it. If this is the predominant factor, then the compound may be highly soluble in water. Most ionic compounds are soluble in water because the electrostatic forces of the polar water molecules are stronger than the electrostatic forces keeping. It decreases the electrostatic forces of attraction, resulting in free ions in aqueous solution. Take 2ml of sample in test tube and take 2ml of distilled water in another tube as control. This is the most soluble compound. Despite these few limitations, water's ability do dissolve ionic compounds is one of the major reasons it is so vital to life on earth. The higher the iodine number, the more c=c bonds are present in the fat (mbatchou and kosoono oils may also be extracted from plants by dissolving parts of plants in water or another solvent. 2 solutions the majority component of a solution is called the solvent. Water is a polar covalent compound. Benzaldehyde and cyclohexanone are both not very soluble in water because of their relatively large carbon chains cyclohexanone is slightly more soluble though, probably because it has one less carbon atom in the carbon chain so slightly weaker van der waals. More trending news?visit yahoo home. Water breaks the ionic bond by hydrogen bonding, as, water itself has a more. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Therefore, it becomes soluble in water.
Solutions Molarity And Intermolecular Forces Chemistry Video Clutch Prep
Distribution Of Iodine Between Two Immiscible Solvents Youtube. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Iodine is not soluble in water because iodine is nonpolar and water is polar. More trending news?visit yahoo home. Get more help from chegg. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Iodine plays an important role in the. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. What are the health effects of iodine in water? Iodine is soluble in both ethanol and hexane. Glucose is very polar and dissolves in polar water.
Chemistry Department Florida State University
How Would I Be Able To Differentiate A Purple Solution Of Potassium Permanganate And A Purple Solution Of Iodine Crystals Quora. What are the health effects of iodine in water? Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Get more help from chegg. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. Iodine is not soluble in water because iodine is nonpolar and water is polar. More trending news?visit yahoo home. Iodine plays an important role in the. Iodine is soluble in both ethanol and hexane. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Glucose is very polar and dissolves in polar water. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2.
Absorption Spectrum Of A 5 10 5 M Molecular Iodine In N Hexane And Download Scientific Diagram
Solved For Each Alcohol Draw The Structure Identify The Chegg Com. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Iodine plays an important role in the. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Get more help from chegg. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. More trending news?visit yahoo home. Iodine is soluble in both ethanol and hexane. What are the health effects of iodine in water? Glucose is very polar and dissolves in polar water. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Iodine is not soluble in water because iodine is nonpolar and water is polar.
Solutions Like Dissolves Like Solubility And Intermolecular Forces Chemdemos
Iodine Wikipedia. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. More trending news?visit yahoo home. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. What are the health effects of iodine in water? Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Get more help from chegg. Glucose is very polar and dissolves in polar water. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Iodine plays an important role in the. Iodine is soluble in both ethanol and hexane. Iodine is not soluble in water because iodine is nonpolar and water is polar.
Determination Of The Equilibrium Constant For The Formation Of Tri Io
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcszcgt4b 1kikdyjerlievkaieauheo3dzjta Usqp Cau. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Iodine plays an important role in the. What are the health effects of iodine in water? Iodine is soluble in both ethanol and hexane. Glucose is very polar and dissolves in polar water. Iodine is not soluble in water because iodine is nonpolar and water is polar. Get more help from chegg. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. More trending news?visit yahoo home. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2.
Olsg Ch111 Solutions
Partition Coefficient Edited Solvent Solubility. Get more help from chegg. What are the health effects of iodine in water? Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. Iodine is soluble in both ethanol and hexane. Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Iodine plays an important role in the. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Glucose is very polar and dissolves in polar water. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. More trending news?visit yahoo home. Iodine is not soluble in water because iodine is nonpolar and water is polar.
Which Compound S Water Acetone Ethanol Hexane Decane 1 Decanol Sodium Chloride And Or Iodine Do You Think Will Homeworklib
Solved 8 Factors That Affect Solubility Of Gases Liquid Chegg Com. Polarity is defined by the partial charges on the molecule that are caused by electronegativity on the constituant. Iodine plays an important role in the. Water solubility of iodine is determined by temperature (20oc) and pressure (1 bar), and is relatively low. Iodine is not soluble in water because iodine is nonpolar and water is polar. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. More trending news?visit yahoo home. Glucose is very polar and dissolves in polar water. According to the like dissolve like expression, nonpolar substances no hexane is insoluble in water. What are the health effects of iodine in water? Iodine is non polar and hence dissolves in non polar solvent hexane and not in polar solvent water. Iodine is better soluble in iodine for more information on chernobyl, see environmental disasters page. Iodine is soluble in both ethanol and hexane. Get more help from chegg. Iodine is covalent in nature and therefore, it does not dissolve in water which is polar. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other.