Is Halogens More Soluble In Water Or Hexane. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. You are given br2 water and i2 water; The color of both solutions is very similar. Similarly one may ask, what is. The halogens are much more soluble in organic solvents like hexane than they are in water. However the bottles are not labeled. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. Amine can make hydrogen bonds with water. Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. By far, the most important criterion for water solubility is hydrogen bonding. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. In hexane, the halogens are more soluble than are the halides. Are halogens more soluble in hexane or water? Reactions between halogens and halides.
Atomic And Physical Properties Of Periodic Table Group 7 The Halogens
Solutions. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. The color of both solutions is very similar. In hexane, the halogens are more soluble than are the halides. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. By far, the most important criterion for water solubility is hydrogen bonding. However the bottles are not labeled. Are halogens more soluble in hexane or water? Reactions between halogens and halides. Similarly one may ask, what is. Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. The halogens are much more soluble in organic solvents like hexane than they are in water. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. You are given br2 water and i2 water; Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. Amine can make hydrogen bonds with water.
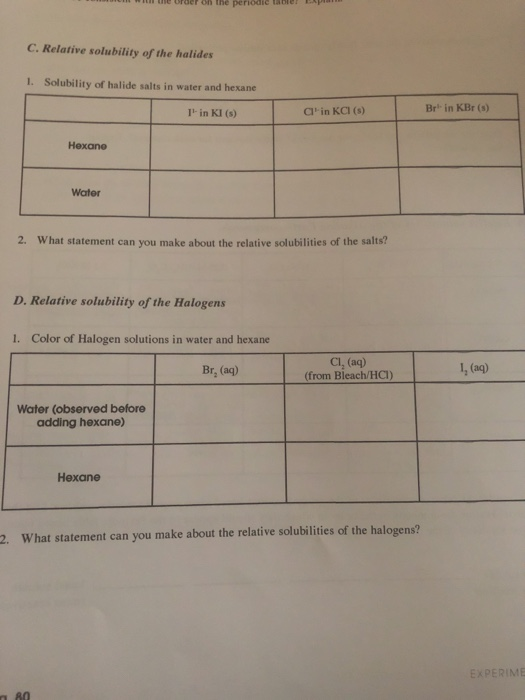
Reactions between halogens and halides.
Amine which have less molecular mass are soluble in water. The halogens also form a number of polyatomic anions, the most important of which are the ions the hexane does not react with the halogens; Which of the following intermolecular forces will exist in the system? There are a number of patterns in the data obtained from measuring the solubility of different salts. For ni(oh)2, adding h^+ reacts with oh to form h2o so yes, it. In hexane, the halogens are more soluble than are the halides. Are halogens more or less soluble in organic solvents than water? Hexane is neither polar not it contain any electronegative element like oxygen , fluorine, nitrogen so it is not hexane is soluble in benzenebecause benzene is also a non polar compound. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Suppose that nacl is added to hexane instead of water. This demonstrates that despite having a polar hydroxyl group that can form hydrogen bonds, alcohols become less soluble in water (and more soluble in nonpolar solvents) as the alkyl portion of the alcohol becomes larger. For agi the acid would be hi and that is a strong acid; Water is a polar covalent compound. Which is more water soluble and why? It have been shown before that dichloromethane is one of the solvent to extract caffeine, but dichloromethane is less polar than other solvents like water, ethyl acetate and acetone, so why is caffeine most soluble in dichloromethane? Amine which have less molecular mass are soluble in water. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. Discover everything scribd has to offer, including books and to test relative solubility in water and hexane: What makes dichloromethane such a good organic. Water and aqueous solutions such as urine will not dissolve such a film, which explains why petroleum jelly protects recall that halogens are the elements in family 7a on the periodic table and contain alkanes (both normal and cycloalkanes) are virtually insoluble in water but dissolve in organic solvents. Amine can make hydrogen bonds with water. Methyl alcohol was soluble in water and partially soluble in hexane. Similarly one may ask, what is. When solids dissolve in water, they dissociate to give the elementary particles from which they are formed. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. The color of both solutions is very similar. Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. It decreases the electrostatic forces of attraction, resulting in free ions in aqueous solution. In alcohol, coconut oil is more soluble than most common fats and oils. Logically it sounds like a phenol should be highly soluble in water. To chemists, the salts are very useful in analysis of.
Atomic And Physical Properties Of Halogens Chemistry Libretexts
Alkyl Halides Preparing Reactions Physical Properties. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. Similarly one may ask, what is. The color of both solutions is very similar. By far, the most important criterion for water solubility is hydrogen bonding. Are halogens more soluble in hexane or water? Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. In hexane, the halogens are more soluble than are the halides. The halogens are much more soluble in organic solvents like hexane than they are in water. Reactions between halogens and halides. Amine can make hydrogen bonds with water. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. However the bottles are not labeled. You are given br2 water and i2 water;
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts
Polar Protic Polar Aprotic Nonpolar All About Solvents. Are halogens more soluble in hexane or water? Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. Similarly one may ask, what is. Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. Amine can make hydrogen bonds with water. In hexane, the halogens are more soluble than are the halides. By far, the most important criterion for water solubility is hydrogen bonding. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. Reactions between halogens and halides. You are given br2 water and i2 water; However the bottles are not labeled. The color of both solutions is very similar. The halogens are much more soluble in organic solvents like hexane than they are in water. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid.
Lab Background
Part B The Operations In This Part Will Take Place In The Hood To Avoid Traffic Homeworklib. Similarly one may ask, what is. The halogens are much more soluble in organic solvents like hexane than they are in water. Amine can make hydrogen bonds with water. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. By far, the most important criterion for water solubility is hydrogen bonding. You are given br2 water and i2 water; Are halogens more soluble in hexane or water? Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. Reactions between halogens and halides. In hexane, the halogens are more soluble than are the halides. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. However the bottles are not labeled. The color of both solutions is very similar.
Chemistry Lower Secondary Ydp Whiteboard Exercise Solubility Of Halogens In Water And In Hexane
Solved î M 2 S Therefore Mi M2 And Mt Mt For Examp Chegg Com. Are halogens more soluble in hexane or water? You are given br2 water and i2 water; Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. The halogens are much more soluble in organic solvents like hexane than they are in water. By far, the most important criterion for water solubility is hydrogen bonding. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. The color of both solutions is very similar. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. Amine can make hydrogen bonds with water. In hexane, the halogens are more soluble than are the halides. Similarly one may ask, what is. Reactions between halogens and halides. However the bottles are not labeled.
Lab Report 1 Chm131 Chemistry Studocu
Supplemental Topics. The color of both solutions is very similar. Amine can make hydrogen bonds with water. Reactions between halogens and halides. However the bottles are not labeled. The halogens are much more soluble in organic solvents like hexane than they are in water. Are halogens more soluble in hexane or water? Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. Similarly one may ask, what is. Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. By far, the most important criterion for water solubility is hydrogen bonding. You are given br2 water and i2 water; Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. In hexane, the halogens are more soluble than are the halides.
Halogens In Aqueous Solution And Their Displacement Reactions Experiment Rsc Education
The Halogens Fluorine Chlorine Bromine Iodine And Astatine Sciencedirect. However the bottles are not labeled. Are halogens more soluble in hexane or water? Similarly one may ask, what is. Reactions between halogens and halides. You are given br2 water and i2 water; Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. The color of both solutions is very similar. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. By far, the most important criterion for water solubility is hydrogen bonding. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. Amine can make hydrogen bonds with water. The halogens are much more soluble in organic solvents like hexane than they are in water. In hexane, the halogens are more soluble than are the halides.
Lab Report 1 Chm131 Chemistry Studocu
Fdyqlppl1ccznm. The color of both solutions is very similar. I have never agreed with the commonly stated principle that polar compounds will be soluble in water since water is polar. It is more soluble as hexane is non polar and potassium manganate is also non polar substances with like polarities mix so potassium manganate is more soluble in hexane. Reactions between halogens and halides. Benzene is not soluble in water because benzene cannot make strong intermolecular forces such as hydrogen bonds or dipole dipole interactions. The halogens are much more soluble in organic solvents like hexane than they are in water. Water and hexane solvent are immiscible, that is, they are insoluble in each other, and cl 2 i 2 hexane red clear pink water pale yellow clear clear b. However the bottles are not labeled. Water is a polar molecule, as are all halogens as exemplified by the simplest common halogen of all, hydrochloric acid. In hexane, the halogens are more soluble than are the halides. You are given br2 water and i2 water; Similarly one may ask, what is. Amine can make hydrogen bonds with water. By far, the most important criterion for water solubility is hydrogen bonding. Are halogens more soluble in hexane or water?