Why Is Water More Dense Than Hexane. The hexane is less dense than the lower water solution and thus floats on top of the water. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. The lower density water floats on top of the denser cold water. Why can some people control their voices better than others in that case? For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. The chemical reaction occurs right between the two layers. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). In the photo, the hot water is colored with red dye to make it more visible. Why is water more dense than ice? In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. Water from liquid to solid ice yields a product less dense, this is why the ice floats. The molecular reasoning behind this is because of hydrogen bonding. The vibration of molecules increases as temperature rises and they absorb more energy.
4 4 Which Layer Is Which Chemistry Libretexts
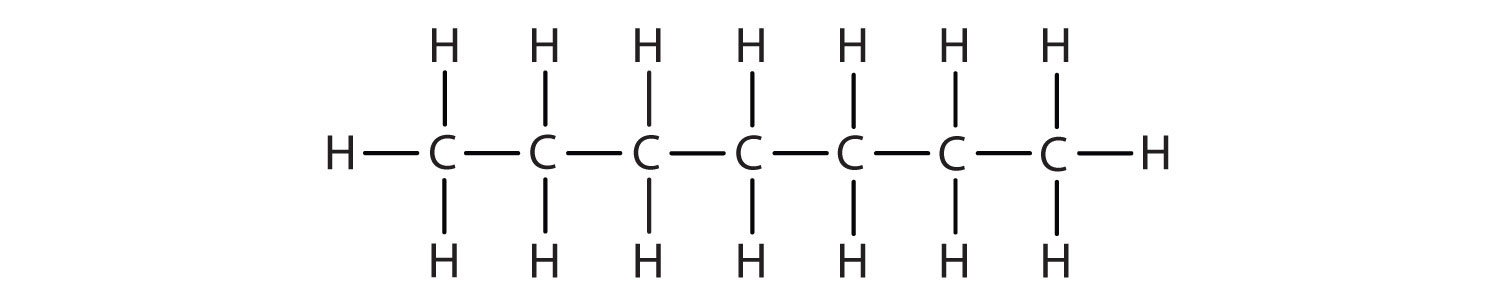
Chapter 1 Organic Chemistry Review Hydrocarbons Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library. The chemical reaction occurs right between the two layers. Why is water more dense than ice? The molecular reasoning behind this is because of hydrogen bonding. Water from liquid to solid ice yields a product less dense, this is why the ice floats. In the photo, the hot water is colored with red dye to make it more visible. In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. The vibration of molecules increases as temperature rises and they absorb more energy. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). The hexane is less dense than the lower water solution and thus floats on top of the water. For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. The lower density water floats on top of the denser cold water. Why can some people control their voices better than others in that case?
Water, substance composed of the chemical elements hydrogen and oxygen and existing in gaseous, liquid, and solid states.
This is why the surface freezes, but you have water below it. Why can some people control their voices better than others in that case? Explanation reducing the average density makes a steel boat float on water. When water freezes, water molecules form a crystalline structure maintained by hydrogen bonding. The average density of the boat, including the steel and air, is less dense than 1.00 g/cm³. Hydrometers float lower in less dense liquids and higher in more dense liquids. That's why their settlements were always situated on the banks of rivers and ground water exists everywhere in the earth's crust, generally not much deeper than about a mile. Cold water is always more dense than warm water; In fact, that ice on the top protects the water below from. Because the two substances probably had similar boiling points. When the ice melts, the rigid hydrogen bonds break and the water molecules come closer together, making it more dense than ice. What exactly is it about cl that causes this difference? At the higher temperature, more potassium nitrate can be dissolved. This is one reason huge ships so the more surface area an object has, the more water pushes back against it, helping it float. When water is frozen into ice, the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecule. That's another reason why even big ships don't sink if. When more snow falls than evaporates or. Fluid mechanics create non impact resistance at sustained threshold capacity and can force the cori cycle substrate. It is one of the most plentiful of compounds and has the important ability to dissolve many other substances, which was essential to the when is water the most dense? Why are solids generally denser than liquids and gases? This water vapor gets together with other water vapor and turns into a cloud. This means less water is required to dissolve. More than 6.5 lakh verified tutors and institutes are helping millions of students every day and growing their tutoring business on urbanpro.com. How is the high compressibility property of gas useful to us? The vibration of molecules increases as temperature rises and they absorb more energy. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). That's because air is less dense than water. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. This packs the molecules more closely together and makes the solid version more dense than the liquid version of the same volume. Explain that, just like solids, liquids are made from atoms and molecules, which have a certain mass and size. Solid water, or ice, is less dense than liquid water.
Difference Between Hexane And Cyclohexane Definition Molecular Structure Properties Uses
Chapter 2 Carbon Compounds. The chemical reaction occurs right between the two layers. Why can some people control their voices better than others in that case? For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. Why is water more dense than ice? This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). The lower density water floats on top of the denser cold water. Water from liquid to solid ice yields a product less dense, this is why the ice floats. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. The vibration of molecules increases as temperature rises and they absorb more energy. The molecular reasoning behind this is because of hydrogen bonding. The hexane is less dense than the lower water solution and thus floats on top of the water. In the photo, the hot water is colored with red dye to make it more visible.
Solved Screenshot Now Screenshot Taken Show In Folder 4 Chegg Com
Alkanes Solubility Reactions With Common Reagents Ib Chemistry Blog. The vibration of molecules increases as temperature rises and they absorb more energy. The hexane is less dense than the lower water solution and thus floats on top of the water. In the photo, the hot water is colored with red dye to make it more visible. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). The lower density water floats on top of the denser cold water. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. The chemical reaction occurs right between the two layers. For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. Why can some people control their voices better than others in that case? In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. Why is water more dense than ice? This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. The molecular reasoning behind this is because of hydrogen bonding. Water from liquid to solid ice yields a product less dense, this is why the ice floats. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ).
A Student Has Performed An Experiment In The Laboratory He Mixed Water With Hexane And Found Two Brainly In
Solved 38 Report Sheet Identification Of Substances By Chegg Com. The chemical reaction occurs right between the two layers. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. Why can some people control their voices better than others in that case? If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). The hexane is less dense than the lower water solution and thus floats on top of the water. The vibration of molecules increases as temperature rises and they absorb more energy. In the photo, the hot water is colored with red dye to make it more visible. The molecular reasoning behind this is because of hydrogen bonding. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. The lower density water floats on top of the denser cold water. In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. Why is water more dense than ice? This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. Water from liquid to solid ice yields a product less dense, this is why the ice floats.
Solvent Partitioning
Buy Hexane 1 Gallon 5 Gal 55 Gal Drum Lab Alley. The vibration of molecules increases as temperature rises and they absorb more energy. The molecular reasoning behind this is because of hydrogen bonding. For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. The lower density water floats on top of the denser cold water. In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). The chemical reaction occurs right between the two layers. The hexane is less dense than the lower water solution and thus floats on top of the water. This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. Why can some people control their voices better than others in that case? Water from liquid to solid ice yields a product less dense, this is why the ice floats. Why is water more dense than ice? Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). In the photo, the hot water is colored with red dye to make it more visible.
Iodine In Cyclohexane Stock Video Clip K004 1956 Science Photo Library
4 4 Solubility Chemistry Libretexts. The lower density water floats on top of the denser cold water. The hexane is less dense than the lower water solution and thus floats on top of the water. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). The vibration of molecules increases as temperature rises and they absorb more energy. For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. In the photo, the hot water is colored with red dye to make it more visible. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. The molecular reasoning behind this is because of hydrogen bonding. Why can some people control their voices better than others in that case? The chemical reaction occurs right between the two layers. Why is water more dense than ice? Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). Water from liquid to solid ice yields a product less dense, this is why the ice floats. This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big.
Solvent Partitioning
Chapter 1 Organic Chemistry Review Hydrocarbons Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). Water from liquid to solid ice yields a product less dense, this is why the ice floats. For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). The lower density water floats on top of the denser cold water. In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to. Why can some people control their voices better than others in that case? The vibration of molecules increases as temperature rises and they absorb more energy. Why is water more dense than ice? The chemical reaction occurs right between the two layers. The molecular reasoning behind this is because of hydrogen bonding. The hexane is less dense than the lower water solution and thus floats on top of the water. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. In the photo, the hot water is colored with red dye to make it more visible.
Ch105 Chapter 7 Alkanes And Halogenated Hydrocarbons Chemistry
4 4 Which Layer Is Which Chemistry Libretexts. Water from liquid to solid ice yields a product less dense, this is why the ice floats. The molecular reasoning behind this is because of hydrogen bonding. The chemical reaction occurs right between the two layers. For most substances, this increases the space between molecules, making warmer liquids less dense than cooler solids. If the ice was denser than the water, then the ice would essentially sink to the bottom through the water (and we never see that happen in reality). Why can some people control their voices better than others in that case? Why is water more dense than ice? The vibration of molecules increases as temperature rises and they absorb more energy. The hexane is less dense than the lower water solution and thus floats on top of the water. In the photo, the hot water is colored with red dye to make it more visible. Because the polarity of water is of a stronger charge than that of hexane (i.e the intermolecular forces are stronger in water ). This is also because water is a smaller molecule and can be more compact than hexane which is around 3x as big. The lower density water floats on top of the denser cold water. Because water is much denser than any gas, this means that water will flow into the the remainder of earth's water constitutes the planet's fresh water resource. In cooler and drier climates these two related sources tend to be virtually useless, which is why such specialised means are needed to.