Why Is Hexane A Nonpolar Molecule. How to determine polarity in a molecule. Some other compounds which are not soluble in. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. It looks like a mirror image of itself. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. Hexane is something like this. The water molecules are better off interacting with themselves. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Alcohol is an intermediately polar molecule. Hexane is a nonpolar molecule. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them.
Solved For The Molecules Below Choose Which Of The Liste Chegg Com
Solved The Solubility Of Hcl Gas In Water A Polar Solven Chegg Com. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. Some other compounds which are not soluble in. Alcohol is an intermediately polar molecule. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. The water molecules are better off interacting with themselves. It looks like a mirror image of itself. Hexane is a nonpolar molecule. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. How to determine polarity in a molecule. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. Hexane is something like this. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol.
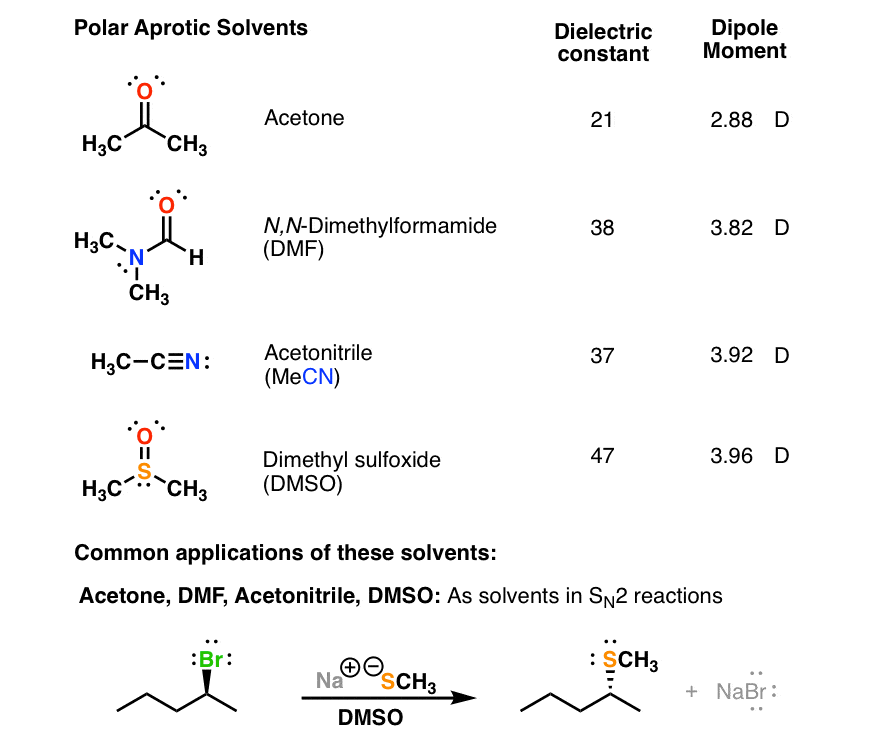
How to determine polarity in a molecule.
A compound can have polar covalent bonds and yet still not be a polar compound. 0.602 g of acetic acid (ch3cooh) were. Polarity describes the distribution of electrical charge around a molecule. Polar covalent molecules exist whenever there is an asymmetry, or uneven distribution of electrons in a molecule. These occur between a polar molecule and a nonpolar molecule, and thus must describe solutions. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. The water molecules are better off interacting with themselves. The effect of the hydroxyl group is quite startling with respect to volatility. Chemical and physical properties of the propane molecule. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. Hexane is a nonpolar molecule. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density nonpolar covalent bonds, with equal sharing of the bond electrons, arise when the electronegativities of the two atoms are equal. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. Oil is nonpolar while water is polar. Octanol, a mostly nonpolar molecule, dissolves in hexane but formed a separate layer in water. Finally biphenyl, a very nonpolar molecule, was found to be soluble in hexane but insoluble in water and methyl alcohol. One or more of these asymmetric atoms pulls electrons more strongly than the other atoms. The freezing point of pure cyclohexane was measured and found to be 6.5 ºc. Benzophenone is more soluble in methanol because both molecules possess some polarity. So this molecule being majorly nonpolar, why would it be more soluble in metoh than in hexane? This demonstrates that despite having a polar hydroxyl group that can form hydrogen bonds, alcohols become less soluble in water (and more soluble in nonpolar solvents) as. A compound can have polar covalent bonds and yet still not be a polar compound. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. Some other compounds which are not soluble in. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. Because alkane molecules are nonpolar, they are insoluble in water, which is a polar solvent, but are nearly all alkanes have densities less than 1.0 g/ml and are therefore less dense than water (the density of h2o is 1.00 g/ml at 20°c). It's helpful to know which compounds are intermediate between polar and. Polar and nonpolar molecules are the two broad classes of molecules. Answer to question 2 (1 point) hexane is a (polar/nonpolar) molecule. It looks like a mirror image of itself. By contrast, a nonpolar molecule has a fairly uniform charge on its outside surface, making no side more negative or positive than another.
Chapter 4 Water Its Effect On Dissolved Biomolecules
Solubility Lab Mini Report 0106 253 Studocu. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. Alcohol is an intermediately polar molecule. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. It looks like a mirror image of itself. Hexane is a nonpolar molecule. Some other compounds which are not soluble in. How to determine polarity in a molecule. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. Hexane is something like this. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. The water molecules are better off interacting with themselves.
Why Is Hexane Nonpolar Quora
Solubility Lab Mini Report 0106 253 Studocu. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. It looks like a mirror image of itself. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. Some other compounds which are not soluble in. Hexane is something like this. Alcohol is an intermediately polar molecule. Hexane is a nonpolar molecule. The water molecules are better off interacting with themselves. How to determine polarity in a molecule. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure.
Polar And Non Polar Solubility
How Is Molecular Polarity Related To Solubility Socratic. The water molecules are better off interacting with themselves. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Some other compounds which are not soluble in. It looks like a mirror image of itself. Hexane is a nonpolar molecule. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. Hexane is something like this. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. How to determine polarity in a molecule. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. Alcohol is an intermediately polar molecule.
Why Is Hexane Nonpolar Quora
4 4 Solubility Chemistry Libretexts. The water molecules are better off interacting with themselves. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. Alcohol is an intermediately polar molecule. Hexane is a nonpolar molecule. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. Some other compounds which are not soluble in. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. Hexane is something like this. How to determine polarity in a molecule. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. It looks like a mirror image of itself. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them.
Is Hexane Polar Or Nonpolar Science Trends
Solved Activity A Polarity Get The Gizmo Ready Check T Chegg Com. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. Hexane is a nonpolar molecule. How to determine polarity in a molecule. The water molecules are better off interacting with themselves. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. Hexane is something like this. Some other compounds which are not soluble in. Alcohol is an intermediately polar molecule. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. It looks like a mirror image of itself. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol.
Why Is Hexane Insoluble In Water Quora
Polar Vs Nonpolar Compounds Demonstration Sheet. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. Alcohol is an intermediately polar molecule. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. The water molecules are better off interacting with themselves. How to determine polarity in a molecule. Some other compounds which are not soluble in. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. It looks like a mirror image of itself. Hexane is something like this. Hexane is a nonpolar molecule. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end.
Solutions And Attractive Forces
Which Solvent Water Or Hexane C6h14 Wo Clutch Prep. The reasons why hexane and water do not mix are complex, but the following gives you a glimpse at why hexane is insoluble in water. * molecules come in infinite varieties, so in order to help the complicated chemical world make a little more sense, we classify and categorize them. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Hexane (c6 h14) is nonpolar becasue of its symmetrcial structure. It is a chain of 6 c atoms in a line, with two h atoms each above and below each one, and at h each end. By the chemistry principal of similar compounds being able to dissolve one another hexane is soluble in. Some other compounds which are not soluble in. Alcohol is an intermediately polar molecule. How to determine polarity in a molecule. When the nonpolar pentane molecules move into the nonpolar hexane, london forces are disrupted between the hexane molecules, but new london forces are. There is no reason for electrons to be concentrated on one side of the molecule, and therefore. Hexane is a nonpolar molecule. Hexane is something like this. The water molecules are better off interacting with themselves. It looks like a mirror image of itself.