Which Is More Soluble In Water Hexane (C6H14) Or Hexanol (C6H13Oh). A solution is a homogeneous mixture of two or more substances. Vitamin c, a compound with several oh groups. Diferentiate between gas and vapour latent heat of vapourisation is used to; What type of solvent would dissolve the other compounds? (a) overcome the ssertion (a): Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. Between between benzene and ethanol , benzene is the most soluble in hexane. The more carbons you add to a molecule the. Butanal is more soluble in water because it has less carbons attached. Reason been benzene can form a hydrogen bond with hexane, also answer: Each hexanol (actually there are three structural isomers: Hexane is a significant constituent of gasoline. Hexane is not a polar molecule, and thus is not soluble in water. A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the.
Chemistry 2202 Chemical Bonding Review Answer The Following In
General Chemistry The Essential Concepts 6th Edition Part 4 By Shidae Hane Issuu. Hexane is not a polar molecule, and thus is not soluble in water. Each hexanol (actually there are three structural isomers: A solution is a homogeneous mixture of two or more substances. Vitamin c, a compound with several oh groups. What type of solvent would dissolve the other compounds? Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. Between between benzene and ethanol , benzene is the most soluble in hexane. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. Reason been benzene can form a hydrogen bond with hexane, also answer: Diferentiate between gas and vapour latent heat of vapourisation is used to; A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. (a) overcome the ssertion (a): Butanal is more soluble in water because it has less carbons attached. Hexane is a significant constituent of gasoline. The more carbons you add to a molecule the.
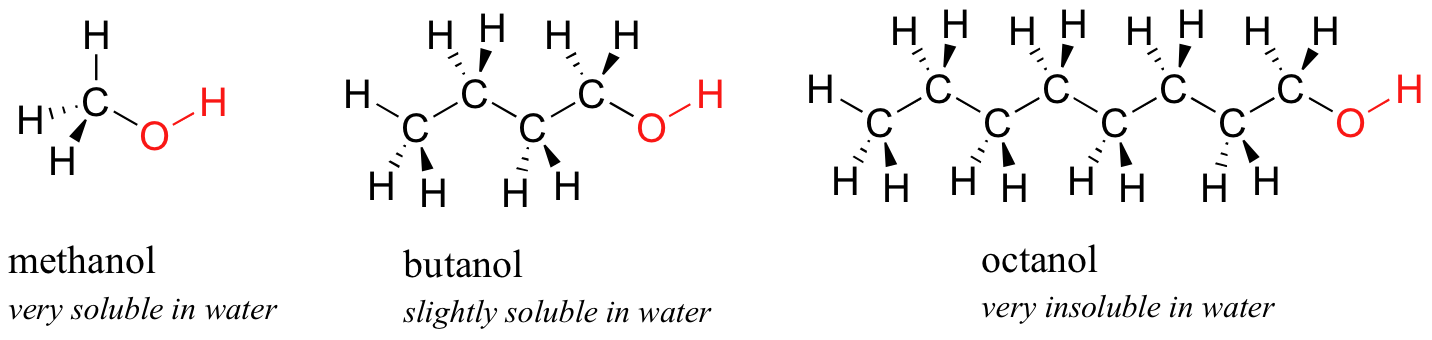
I am sorry for the vague response, yahoo does not.
Solubility slightly soluble in water, freely soluble in. Hexane (c6h14) or hexanol (c6h13oh)? Between between benzene and ethanol , benzene is the most soluble in hexane. The more carbons you add to a molecule the. The amounts are in mol/100g of h2o at 1atm and 25oc. I know this may sound pretty idiotic, and i only have a basic understanding of solubility, but technically, since water surrounds itself with water when it is mixed with more water, does that make it soluble within itself? With a polar compound, water would be a safer option than hexane, because water is polar and can interact. (shorter chain = more soluble) a. The longer it is, the less soluble it will be in water. The answer is supposedly cyclohexanol. A) hexane, because it is more hydrophobic. Cloth fibers swell when they are washed in water. Primary alcohol > secondary alcohol > tertiary alcohol, due to decrease in polar character. As the solvent becomes more nonpolar, the solubility of this polar solute decreases. It has to do with bonding formations, molecular weight, and structure. Vitamin c, a compound with several oh groups. The dissolution of the following in water or hexane has to be predicted. Hexanes chemical formula c 6 h 14 formula weight description clear colourless highly flammable liquid with a characteristic a beer s law experiment introduction there are many ways to determine concentrations of a substance in solution. (a) overcome the ssertion (a): Many isomeric alcohols have the formula c6h13oh. Ch3oh would form more of a suqare bond. How to draw cyclohexane chair conformations and ring flips. 2 ethylhexanol solubility in water. As water is polar it attracts oh group. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Whether you need hplc hexane, acs hexane, anhydrous hexane or one of the other grades available, we offer the right product for your application. Using what you know of hydrophilic and hydrophobic solutes, classify each as water soluble or fat soluble and predict which are likely to be required in the diet on. Diferentiate between gas and vapour latent heat of vapourisation is used to; Each hexanol (actually there are three structural isomers: The bonds involving the atoms with various electronegativites having a large dipole moment are called as polar solvents.
Owlbook Ch11 Intermolecular Force Chemical Polarity
Owlbook Ch11 Intermolecular Force Chemical Polarity. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. A solution is a homogeneous mixture of two or more substances. Each hexanol (actually there are three structural isomers: Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. Hexane is a significant constituent of gasoline. Diferentiate between gas and vapour latent heat of vapourisation is used to; What type of solvent would dissolve the other compounds? Reason been benzene can form a hydrogen bond with hexane, also answer: (a) overcome the ssertion (a): A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. The more carbons you add to a molecule the. Hexane is not a polar molecule, and thus is not soluble in water. Butanal is more soluble in water because it has less carbons attached. Between between benzene and ethanol , benzene is the most soluble in hexane. Vitamin c, a compound with several oh groups.
General Chemistry Atoms First Pdf Ion Covalent Bond
Which Solvent Water Or Hexane C6h14 Wo Clutch Prep. Each hexanol (actually there are three structural isomers: Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. Vitamin c, a compound with several oh groups. Hexane is a significant constituent of gasoline. Between between benzene and ethanol , benzene is the most soluble in hexane. The more carbons you add to a molecule the. Diferentiate between gas and vapour latent heat of vapourisation is used to; A solution is a homogeneous mixture of two or more substances. (a) overcome the ssertion (a): What type of solvent would dissolve the other compounds? Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. Butanal is more soluble in water because it has less carbons attached. Reason been benzene can form a hydrogen bond with hexane, also answer: Hexane is not a polar molecule, and thus is not soluble in water. A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1.
Biochemistry Bms1049 Flashcards Quizlet
Owlbook Ch11 Intermolecular Force Chemical Polarity. (a) overcome the ssertion (a): What type of solvent would dissolve the other compounds? Between between benzene and ethanol , benzene is the most soluble in hexane. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. Vitamin c, a compound with several oh groups. The more carbons you add to a molecule the. Hexane is a significant constituent of gasoline. Each hexanol (actually there are three structural isomers: Butanal is more soluble in water because it has less carbons attached. Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. A solution is a homogeneous mixture of two or more substances. Diferentiate between gas and vapour latent heat of vapourisation is used to; Reason been benzene can form a hydrogen bond with hexane, also answer: Hexane is not a polar molecule, and thus is not soluble in water.
Solved Which One Of The Following Is Most Soluble In Hexa Chegg Com
Complete Combustion Of Hexane C6h14 Balanced Equation Youtube. Vitamin c, a compound with several oh groups. Hexane is not a polar molecule, and thus is not soluble in water. The more carbons you add to a molecule the. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. (a) overcome the ssertion (a): Each hexanol (actually there are three structural isomers: Hexane is a significant constituent of gasoline. Between between benzene and ethanol , benzene is the most soluble in hexane. Diferentiate between gas and vapour latent heat of vapourisation is used to; A solution is a homogeneous mixture of two or more substances. A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. Reason been benzene can form a hydrogen bond with hexane, also answer: What type of solvent would dissolve the other compounds? Butanal is more soluble in water because it has less carbons attached.
Which Solvent Water Or Hexane C6h14 Wo Clutch Prep
Chemistry 2202 Chemical Bonding Review Answer The Following In. A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. Between between benzene and ethanol , benzene is the most soluble in hexane. Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. Hexane is a significant constituent of gasoline. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. (a) overcome the ssertion (a): The more carbons you add to a molecule the. What type of solvent would dissolve the other compounds? Butanal is more soluble in water because it has less carbons attached. Vitamin c, a compound with several oh groups. Diferentiate between gas and vapour latent heat of vapourisation is used to; A solution is a homogeneous mixture of two or more substances. Reason been benzene can form a hydrogen bond with hexane, also answer: Hexane is not a polar molecule, and thus is not soluble in water. Each hexanol (actually there are three structural isomers:
Carta Resistencia De Materiales Ammonia Calcium
Sort The Following Compounds Based On Whet Clutch Prep. Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. What type of solvent would dissolve the other compounds? The more carbons you add to a molecule the. Each hexanol (actually there are three structural isomers: A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. A solution is a homogeneous mixture of two or more substances. (a) overcome the ssertion (a): Hexane is not a polar molecule, and thus is not soluble in water. Diferentiate between gas and vapour latent heat of vapourisation is used to; Between between benzene and ethanol , benzene is the most soluble in hexane. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. Vitamin c, a compound with several oh groups. Butanal is more soluble in water because it has less carbons attached. Reason been benzene can form a hydrogen bond with hexane, also answer: Hexane is a significant constituent of gasoline.
Hexanol C6h14o Chemspider
Organic Chemistry By Arindam Choudhuri Issuu. Hexane is not a polar molecule, and thus is not soluble in water. Therefore, out of those 3 options the most soluble molecule in water will be ethanol (c2h5oh) because of the oxygen present in the. A solution contains 10g of sugar in 170g of water.calculate to concentration in terms of mass by mass percentage of the solution 1. Whereas c6h13oh has that nice hydroxyl radical added on, which gives the molecule some polarity. Hexane is a significant constituent of gasoline. (a) overcome the ssertion (a): Reason been benzene can form a hydrogen bond with hexane, also answer: The more carbons you add to a molecule the. Butanal is more soluble in water because it has less carbons attached. Vitamin c, a compound with several oh groups. Each hexanol (actually there are three structural isomers: Between between benzene and ethanol , benzene is the most soluble in hexane. What type of solvent would dissolve the other compounds? Diferentiate between gas and vapour latent heat of vapourisation is used to; A solution is a homogeneous mixture of two or more substances.