Which Is More Polar Hexane Or Heptane. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. This organic chemistry video tutorial explains how to determine which bond is more polar. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. More trending news?visit yahoo home. Hexane is a significant constituent of gasoline. Therefore pentane, heptane and octane are equally nonpolar. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. Thus all alkanes have a polarity of 0. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. If you were to add water however. Can't you modify your other phase to get the selectivity you want? Both heptane and hexane fall into the category of alkanes. It also explains how to rank the bonds from least polar to most.
World S Top N Heptane Manufacturers Market Research Reports Inc
Effect Of The Organic Solvent On The Interfacial Micropolarity Of Aot Water Reverse Micelles. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. Thus all alkanes have a polarity of 0. If you were to add water however. Can't you modify your other phase to get the selectivity you want? Both heptane and hexane fall into the category of alkanes. Hexane is a significant constituent of gasoline. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. More trending news?visit yahoo home. Therefore pentane, heptane and octane are equally nonpolar. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. It also explains how to rank the bonds from least polar to most. This organic chemistry video tutorial explains how to determine which bond is more polar. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up.
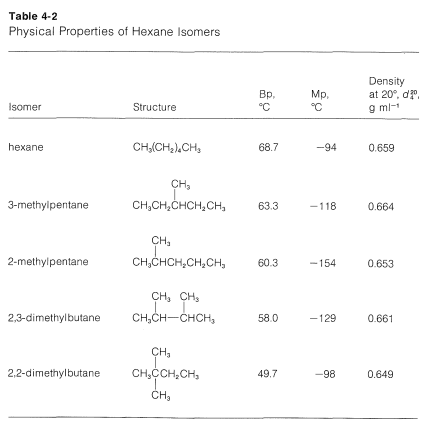
Adjusting polarity that way is easier than trying to.
If you want to use hexane i would buy a good grade, or refine it through fyi, adsorbent scrubs are significantly more effective after removal of phosphatides. Liquid mercury solid copper hexane, a nonpolar solvent. Therefore pentane, heptane and octane are equally nonpolar. However, blocking some types of cookies may impact your experience of the site and the services we are able to offer. Though hexane is commonly used in cleaning agents, high levels of hexane exposure. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Heptane is a better choice over hexane, you can find it cleaner/cheaper. Matthias wants to dissolve a solid sample of calcium chloride (cacl2), which is an ionic compound. I thought that ethyl acetate/hexane was polar so i would have thought caffeine would be the least polar since it traveled the shortest. If you were to add water however. Hexane has less number of isomers than heptane. The need to repeat the extraction on the other suitable solvents can include pentane (very volatile) cyclohexane,heptane and isooctane. Hexane is also often used as a solvent with many. Hexane occurs in a couple of different places in nature, but is usually most readily available in petroleum deposits. C.h is more polar than hexamne.bcoz hexane having symmeyrical td.str.its having 0 dipole moment.while in d case of cyclo hexane its havng. Thus all alkanes have a polarity of 0. For many applications one also can use petroleum ether (ligroin), which is available with different boiling trajectories. Hexane is a significant constituent of gasoline. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. Octanol is less polar than ethanol. Can't you modify your other phase to get the selectivity you want? Hexane and heptane are miscible with each other in all proportions. Hexane and heptane have 5 and 9 isomers respectively. In making a solution of them, is the entropy of the system increased, decreased, or close to zero, compared to the separate pure get more help from chegg. Also, disassociation of said phosphatides with low ph saline is crucial. Adjusting polarity that way is easier than trying to. Which solvent will dissolve the sample of calcium chloride? Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. Both heptane and hexane fall into the category of alkanes. More trending news?visit yahoo home. 3:1:1 hexane/acetone/acetic acid would be considered quite a polar solvent mix.
Understanding The Effect Of Solvent Polarity On The Polymorphism Of Octadecanoic Acid Through Spectroscopic Techniques And Dft Calculations Crystengcomm Rsc Publishing
Solved Table 1 Physical Properties Of Alkanes And Alcoho Chegg Com. Both heptane and hexane fall into the category of alkanes. If you were to add water however. This organic chemistry video tutorial explains how to determine which bond is more polar. Thus all alkanes have a polarity of 0. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. Can't you modify your other phase to get the selectivity you want? Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. It also explains how to rank the bonds from least polar to most. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. More trending news?visit yahoo home. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. Hexane is a significant constituent of gasoline. Therefore pentane, heptane and octane are equally nonpolar.
Effect Of The Organic Solvent On The Interfacial Micropolarity Of Aot Water Reverse Micelles
Medical Marijuana Solvent Extraction Efficiency Potency Determinations With Gc Fid Chromablography Restek S Chromatography Blog. Can't you modify your other phase to get the selectivity you want? Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. This organic chemistry video tutorial explains how to determine which bond is more polar. It also explains how to rank the bonds from least polar to most. If you were to add water however. Therefore pentane, heptane and octane are equally nonpolar. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. Hexane is a significant constituent of gasoline. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. Thus all alkanes have a polarity of 0. More trending news?visit yahoo home. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. Both heptane and hexane fall into the category of alkanes.
Solved Question 17 Consider The Temperature Composition D Chegg Com
Profile On Petroleum Ether Scribe. Hexane is a significant constituent of gasoline. Can't you modify your other phase to get the selectivity you want? This organic chemistry video tutorial explains how to determine which bond is more polar. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. It also explains how to rank the bonds from least polar to most. Therefore pentane, heptane and octane are equally nonpolar. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. More trending news?visit yahoo home. If you were to add water however. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. Both heptane and hexane fall into the category of alkanes. Thus all alkanes have a polarity of 0. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond.
Answered In Each Of The Following Pairs Of Bartleby
Effect Of The Organic Solvent On The Interfacial Micropolarity Of Aot Water Reverse Micelles. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. Can't you modify your other phase to get the selectivity you want? Thus all alkanes have a polarity of 0. It also explains how to rank the bonds from least polar to most. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. More trending news?visit yahoo home. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. Hexane is a significant constituent of gasoline. Both heptane and hexane fall into the category of alkanes. If you were to add water however. This organic chemistry video tutorial explains how to determine which bond is more polar. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Therefore pentane, heptane and octane are equally nonpolar.
I Thought In Hplc Non Polar Has Higher Retention Mcat
World S Top N Heptane Manufacturers Market Research Reports Inc. Thus all alkanes have a polarity of 0. More trending news?visit yahoo home. Therefore pentane, heptane and octane are equally nonpolar. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. It also explains how to rank the bonds from least polar to most. Hexane is a significant constituent of gasoline. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. Can't you modify your other phase to get the selectivity you want? For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. If you were to add water however. This organic chemistry video tutorial explains how to determine which bond is more polar. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. Both heptane and hexane fall into the category of alkanes. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane.
Influence Of Solvent Selection And Extraction Temperature On Yield And Composition Of Lipids Extracted From Spent Coffee Grounds Sciencedirect
Effect Of The Organic Solvent On The Interfacial Micropolarity Of Aot Water Reverse Micelles. Both heptane and hexane fall into the category of alkanes. Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. More trending news?visit yahoo home. Can't you modify your other phase to get the selectivity you want? Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. This organic chemistry video tutorial explains how to determine which bond is more polar. Therefore pentane, heptane and octane are equally nonpolar. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. Hexane is a significant constituent of gasoline. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. Thus all alkanes have a polarity of 0. If you were to add water however. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound. It also explains how to rank the bonds from least polar to most.
Weak Donor Strong Acceptor Linked Anthracenyl P Conjugates As Solvato Fluoro Chromophore And Aeegens Contrast Between Nitro And Cyano Functionality Abstract Europe Pmc
Solved A Chemist Has A Mixture Of Hexane And Heptane A A Chegg Com. Both heptane and hexane fall into the category of alkanes. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. Therefore pentane, heptane and octane are equally nonpolar. Theres more van der waals forces going on with heavier hydrocarbons, so there will be more temporary dipole moments popping up. For a molecule to be polar, there must be a covalent bond formed between two atoms with an electronegativity difference. This organic chemistry video tutorial explains how to determine which bond is more polar. Hexane is a significant constituent of gasoline. Hexane is more volatile (bad) and should have a lower viscosity (good) compared to heptane. Can't you modify your other phase to get the selectivity you want? Hexane and octane is easier to separate than hexane and heptane because the difference in their boiling points is greater thus hexane will distill acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. It also explains how to rank the bonds from least polar to most. Thus all alkanes have a polarity of 0. More trending news?visit yahoo home. If you were to add water however. Where, alkanes are hydrocarbons which contain carbon and hydrogen atoms bound with each heptane is an organic compound containing seven carbon atoms bound to each other forming an alkane while hexane is an organic compound.