Which Is More Polar Hexane Or Ethyl Acetate. Ethyl acetate is more polar than hexane. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. Are either of these solvents effective at separating the mixture by tlc? Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. If you were to add water. Ethyl acetate is more polar than hexane. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Are either of these solvents effective at separating the mixture by tlc? The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? This is because ethanol contains oh groups which are more polar.
Lab 2 Thin Layer And Flash Chromatography Isites
2 3d Separation Theory Chemistry Libretexts. Are either of these solvents effective at separating the mixture by tlc? At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. This is because ethanol contains oh groups which are more polar. Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Ethyl acetate is more polar than hexane. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Ethyl acetate is more polar than hexane. If you were to add water. The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? Are either of these solvents effective at separating the mixture by tlc? Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my.
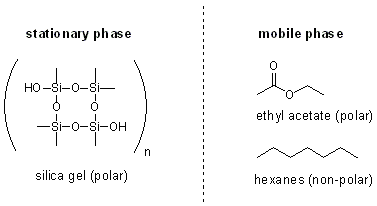
At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent;
Tlc pulls molecules based on their relative polarity to the solvent. Actually ethyl acetate in this case is the most polar solvent, as sometimes called strongest. Earn free access learn more >. Tlc pulls molecules based on their relative polarity to the solvent. Therefore, the stationary phase will always. Ethyl acetate is more polar than hexane. This organic chemistry video tutorial explains how to determine which bond is more polar. Which would you expect to elute from the column first? If you were to add water. Rank each product from least polar to most polar ethyl acetate, hexane, methanol,dichloromethane, the easiest way to tell this. This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers. Ethyl acetate is more polar than hexane. Protocol refer to chm 1321 organic chemistry laboratory manual pages 17 19 observations part a 8 2 ethyl acetate and hexanes solvent 1 benzophenone reference 3. Ethyl acetate is an organic compound that is an ester derived from the combination of ethanol and acetic acid. Acetone is more polar than hexane as it has a net dipole moment. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? Hexane is less polar than ethyl acetate, so a 100% hexane solvent would be less polar than the 20/80% mix. Carbon and hydrogen have an electronegativity difference of 0.35, which classifies the if the difference is greater than 2, then the bond is considered completely polar and is more appropriately called an ionic bond. If you know the polarity of molecules, you can predict whether or not they will mix together to form chemical solutions. Hexane is nonpolar compound as it contains nonpolar carbons. Ethyl acetate (systematically ethyl ethanoate, commonly abbreviated etoac, etac or ea) is the organic compound with the formula ch3−coo−ch2−ch3, simplified to c4h8o2. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; Which is the most polar of each solvent pair? It also explains how to rank the bonds from least polar to most. Are either of these solvents effective at separating the mixture by tlc? This is because ethanol contains oh groups which are more polar. Im using this as a solvent in a tlc experiment. Which is more polar, fluorene or fluorenone? In most cases, hexane/ethyl acetate mixtures work best for me.
Simultaneous Determination Of Active Compounds In Piper Betle Linn Leaf Extract And Effect Of Extracting Solvents On Bioactivity Nguyen 2020 Engineering Reports Wiley Online Library
Plos One Induction Of Apoptosis In Mcf 7 Cells Via Oxidative Stress Generation Mitochondria Dependent And Caspase Independent Pathway By Ethyl Acetate Extract Of Dillenia Suffruticosa And Its Chemical Profile. This is because ethanol contains oh groups which are more polar. If you were to add water. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; Ethyl acetate is more polar than hexane. Are either of these solvents effective at separating the mixture by tlc? The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? Ethyl acetate is more polar than hexane. Are either of these solvents effective at separating the mixture by tlc?
Solvent System Formulation
Chemical Forums Liquid Liquid Extractions What Happens When You Mix Solvents. Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Are either of these solvents effective at separating the mixture by tlc? Ethyl acetate is more polar than hexane. Are either of these solvents effective at separating the mixture by tlc? This is because ethanol contains oh groups which are more polar. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; Ethyl acetate is more polar than hexane. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: If you were to add water.
Solved 6 If You Spot Three Compounds A B And C On A Thi Chegg Com
2 3d Separation Theory Chemistry Libretexts. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Ethyl acetate is more polar than hexane. The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? Ethyl acetate is more polar than hexane. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. Are either of these solvents effective at separating the mixture by tlc? This is because ethanol contains oh groups which are more polar. Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Are either of these solvents effective at separating the mixture by tlc? If you were to add water. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc?
Solved For The Molecules Below Choose Which Of The Liste Chegg Com
2 3d Separation Theory Chemistry Libretexts. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Are either of these solvents effective at separating the mixture by tlc? The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? This is because ethanol contains oh groups which are more polar. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? Ethyl acetate is more polar than hexane. If you were to add water. Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Ethyl acetate is more polar than hexane. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Are either of these solvents effective at separating the mixture by tlc? Ethyl acetate is less polar than ethanol, even though it contains more polar bonds.
Experiment 8
The Silica Gel Is Polar And It Tries To Bind To The Compounds In Stationary Course Hero. This is because ethanol contains oh groups which are more polar. Ethyl acetate is more polar than hexane. Are either of these solvents effective at separating the mixture by tlc? Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? If you were to add water. Ethyl acetate is more polar than hexane. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent; I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Are either of these solvents effective at separating the mixture by tlc? Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my.
Thin Layer Chromatography Tlc Mendelset
Untitled Document. If you were to add water. Are either of these solvents effective at separating the mixture by tlc? Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Ethyl acetate is more polar than hexane. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. This is because ethanol contains oh groups which are more polar. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Ethyl acetate is more polar than hexane. Are either of these solvents effective at separating the mixture by tlc? Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent;
Solved Uuuplu Diu Pli Juhi Uluuur Show Pop Top Creu 1 Chegg Com
A Student Developed A Tlc Plate That Was Spotted With A Mixt Clutch Prep. What would you expect to see if pure hexane had been used to elute the mixture in the column and/or the tlc? The hexane/ethyl acetate/ethanol travelled up the tlc faster, does this make it more polar? I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Ethyl acetate is more polar than hexane. Ethyl acetate is more polar than hexane. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. If you were to add water. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Are either of these solvents effective at separating the mixture by tlc? This is because ethanol contains oh groups which are more polar. Are either of these solvents effective at separating the mixture by tlc? Acetone is slightly more polar than non polar, hexane however is completely non polar making this a non polar solution. At the top of tlc plate and why it depends upon the stationary phase and the mobile phase, to some extent;