Which Example Is An Endothermic Reaction. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. An endothermic reaction is a type of endergonic reaction. However, not all endergonic reactions are endothermic. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. This is the currently selected item. Create your own flashcards or choose from millions created by other students. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Quizlet is the easiest way to study, practise and master what you're learning. That's because you were given two different salts. It doesn't have to be table salt, nor does the solvent need to be water. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. This is an endothermic reaction at play. A good example of an endothermic reaction includes dissolving a salt. When you think about chemical reactions, do you only think about those causing heat? What is an endothermic reaction?
Difference Between Endothermic And Exothermic Reactions Compare The Difference Between Similar Terms
Q9 What Does One Mean By Exothermic And Endothermic Reactions Give Examples Youtube. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. What is an endothermic reaction? When you think about chemical reactions, do you only think about those causing heat? This is the currently selected item. Quizlet is the easiest way to study, practise and master what you're learning. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. It doesn't have to be table salt, nor does the solvent need to be water. However, not all endergonic reactions are endothermic. That's because you were given two different salts. A good example of an endothermic reaction includes dissolving a salt. An endothermic reaction is a type of endergonic reaction. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. Create your own flashcards or choose from millions created by other students. This is an endothermic reaction at play.
D) graphite is more stable than diamond and energy is needed to convert graphite into diamond.
These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Making new bonds is exothermic because energy is released. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Here's a list of examples of endothermic reactions. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. An endothermic reaction is one that absorbs energy in the form of heat or light. The burning of fuel is an example of a combustion reaction, and we as humans rely heavily on this process for our energy requirements. The endothermic reaction of nitrogen and oxygen to form nitric oxide results in a positive change of enthalpy of +180.5 kj. Dehydrogenation of ethyl benzene to form styrene is an endothermic reaction (δh = 188 kj/m). Quizlet is the easiest way to study, practise and master what you're learning. An endothermic reaction is any chemical reaction that absorbs heat from its environment. Many endothermic reactions helps us i our daily life. Demonstration of exothermic and endothermic reactions. C) dehydrogenation is an endothermic reaction since energy is required to break the c−h bonds to convert ethane to ethylene. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The process by which plants make their own food or what is called photosynthesis is a good example of an endothermic reaction. An endothermic reaction is any chemical reaction that takes in heat from its surroundings. D) graphite is more stable than diamond and energy is needed to convert graphite into diamond. Combustion, coal is burnt, mix of cao with water Energy must be absorbed to break a bond, so breaking bonds is endothermic. Energy is used to break bonds in reactants, and energy is released when new bonds form in products. An endothermic reaction is a type of endergonic reaction. However, not all endergonic reactions are endothermic. What is an endothermic reaction? We explain endothermic/exothermic reactions with video tutorials and quizzes, using our many ways(tm) approach from multiple teachers. You can use these when asked to cite an example or to get ideas to set up a demonstration of an endothermic reaction or process. All chemical reactions involve energy. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. A good example of an. Combustion of coal (mainly carbon) in. The beaker now contains sodium ethanoate, water and carbon dioxide, and the thermometer is showing a fall in temperature, so this was an endothermic.
Exothermic And Endothermic Reactions Mr Carson S Science Page
Dublin Schools Lesson Exothermic And Endothermic. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. When you think about chemical reactions, do you only think about those causing heat? Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. An endothermic reaction is a type of endergonic reaction. This is an endothermic reaction at play. This is the currently selected item. Create your own flashcards or choose from millions created by other students. That's because you were given two different salts. Quizlet is the easiest way to study, practise and master what you're learning. It doesn't have to be table salt, nor does the solvent need to be water. What is an endothermic reaction? However, not all endergonic reactions are endothermic. A good example of an endothermic reaction includes dissolving a salt.
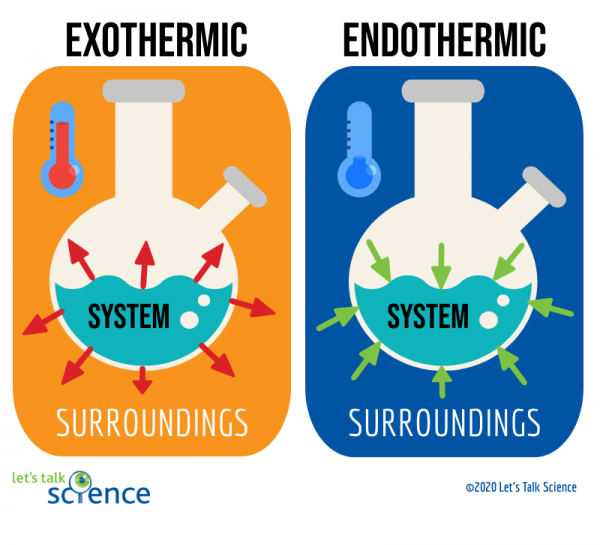
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
Exothermic Reactions. However, not all endergonic reactions are endothermic. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. That's because you were given two different salts. Create your own flashcards or choose from millions created by other students. When you think about chemical reactions, do you only think about those causing heat? Quizlet is the easiest way to study, practise and master what you're learning. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. A good example of an endothermic reaction includes dissolving a salt. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. What is an endothermic reaction? It doesn't have to be table salt, nor does the solvent need to be water. This is an endothermic reaction at play. An endothermic reaction is a type of endergonic reaction. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. This is the currently selected item.
Ks4 Examples Of Endothermic And Exothermic Reactions Teaching Resources
Written Researched And Compiled By Backstroker Ppt Download. That's because you were given two different salts. Quizlet is the easiest way to study, practise and master what you're learning. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. However, not all endergonic reactions are endothermic. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. An endothermic reaction is a type of endergonic reaction. It doesn't have to be table salt, nor does the solvent need to be water. This is an endothermic reaction at play. When you think about chemical reactions, do you only think about those causing heat? Create your own flashcards or choose from millions created by other students. A good example of an endothermic reaction includes dissolving a salt. What is an endothermic reaction? This is the currently selected item.
Exothermic Reaction Read Chemistry Ck 12 Foundation
Dublin Schools Lesson Exothermic And Endothermic. When you think about chemical reactions, do you only think about those causing heat? This is an endothermic reaction at play. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. That's because you were given two different salts. A good example of an endothermic reaction includes dissolving a salt. This is the currently selected item. Quizlet is the easiest way to study, practise and master what you're learning. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. However, not all endergonic reactions are endothermic. It doesn't have to be table salt, nor does the solvent need to be water. Create your own flashcards or choose from millions created by other students. What is an endothermic reaction? An endothermic reaction is a type of endergonic reaction. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water.
What Is An Exothermic Reaction Quora
Exothermic And Endothermic Reactions By Monika Delginova. This is an endothermic reaction at play. An endothermic reaction is a type of endergonic reaction. When you think about chemical reactions, do you only think about those causing heat? Create your own flashcards or choose from millions created by other students. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. That's because you were given two different salts. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. What is an endothermic reaction? It doesn't have to be table salt, nor does the solvent need to be water. This is the currently selected item. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. A good example of an endothermic reaction includes dissolving a salt. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. However, not all endergonic reactions are endothermic. Quizlet is the easiest way to study, practise and master what you're learning.
The Cold Pack A Chilly Example Of An Endothermic Reaction Let S Talk Science
Ks4 Examples Of Endothermic And Exothermic Reactions Teaching Resources. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. When you think about chemical reactions, do you only think about those causing heat? This is an endothermic reaction at play. Create your own flashcards or choose from millions created by other students. This is the currently selected item. It doesn't have to be table salt, nor does the solvent need to be water. That's because you were given two different salts. What is an endothermic reaction? An endothermic reaction is a type of endergonic reaction. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. A good example of an endothermic reaction includes dissolving a salt. However, not all endergonic reactions are endothermic. Quizlet is the easiest way to study, practise and master what you're learning. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.
C2 5 Exothermic And Endothermic Reactions
Chemical Reactions And Energy Ck 12 Foundation. However, not all endergonic reactions are endothermic. When you think about chemical reactions, do you only think about those causing heat? These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Quizlet is the easiest way to study, practise and master what you're learning. If you've ever sprained your ankle or fell from your bike, you've probably reached for an instant cold pack. Create your own flashcards or choose from millions created by other students. This is an endothermic reaction at play. One of your salts generated an endothermic reaction with water, while the other salt generated an exothermic reaction with water. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. What is an endothermic reaction? It doesn't have to be table salt, nor does the solvent need to be water. This is the currently selected item. A good example of an endothermic reaction includes dissolving a salt. That's because you were given two different salts. An endothermic reaction is a type of endergonic reaction.