What Is Hexane Not Soluble In Water. Hexane also does not conduct electricity. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Why is hexane not soluble in water? Hexane is one element that is symbolized by the chemical formula of c6h14. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Water is a polar covalent substance and hexane is nonpolar. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Such element has an alkaline property that consists of six carbon atoms. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Hexane is a significant constituent of gasoline. Hexane is an organic compound used as a solvent. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent.
Olsg Ch111 Solutions
Why Is Cyclohexene Insoluble In Water Quora. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Hexane is one element that is symbolized by the chemical formula of c6h14. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Why is hexane not soluble in water? Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Hexane is an organic compound used as a solvent. Water is a polar covalent substance and hexane is nonpolar. Such element has an alkaline property that consists of six carbon atoms. Hexane also does not conduct electricity. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Hexane is a significant constituent of gasoline. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent.
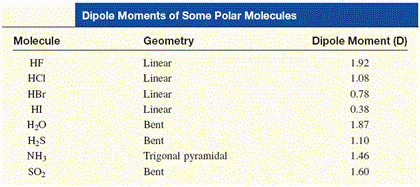
Why is hexane not soluble in water?
Water is a polar covalent substance and hexane is nonpolar. Though hexane is commonly used in cleaning agents, high levels of hexane exposure. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Substances that do dissolve in water won't dissolve any further once they reach saturation point. But i got it wrong. For each of the following molecules, would you expect greater solubility in water or in hexane? Add 120 ml of the buffer solution to both separatory funnels to adjust. If we add hexane to water, the hexane will float on the top of the water with no apparent mixing. Relative strengths of attractive forces. Hexane occurs in a couple of different places in nature. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. It evaporates very easily into the air and dissolves only slightly in water. Soluble in water or soluble in hexane. Water may be known as the universal solvent because it dissolves more substances than any other liquid, but some things won't ever dissolve in water. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Hexane is commonly used to produce pharmaceuticals and even food products, and is not mother's milk, but the point is moot, as it is not left in amounts that are toxic. Whenever this salt is put into water which has two positive dipole moments (one at each hydrogen) and one negative dipole moment (on the oxygen) the negatives and positives of these substances interact which dissolves the substance. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Iodine is soluble in both ethanol and hexane. Water is the solvent, and ethanol is the solute. At first , it is con fusing that what are you talking about. Many organic compounds are not very soluble in water, however, solubility is really a continuous property of chemicals there is no real cut off point that means one chemical is soluble and another is not soluble. Hexane also does not conduct electricity. Water is a polar covalent substance and hexane is nonpolar. Hexanes prepared at the 51st jecfa (1998), published in fnp 52 add 6 (1998) superseding specifications prepared at the 14th jecfa dilute to the mark with water and drain the contents of the flask into a separatory funnel. Suppose that nacl is added to hexane instead of water. Moving to ease of use, because of its insolubility in water, you can soak plant material in hexane without picking up water solubles. Ionic compounds dissolve in water if the energy given off when the ions interact with water molecules compensates for the energy needed to break the ionic bonds in the solid and the energy required to separate the water molecules so that the ions can be inserted into solution. Insoluble in water, soluble in ether, alcohol, and acetone. A solution contains 48.6 g glucose (c6h12o6) dissolved in 0.800 l of water. All orgaic compounds of alkanes, alkenes, alkynes, alkyl halides habe the lowest solubility in water.
Solubility Of Iodine In A Water And Hexane Mixture Please Help The Student Room
Solubility And Molecular Structure. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Why is hexane not soluble in water? Hexane also does not conduct electricity. Hexane is an organic compound used as a solvent. Such element has an alkaline property that consists of six carbon atoms. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Hexane is a significant constituent of gasoline. Hexane is one element that is symbolized by the chemical formula of c6h14. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Water is a polar covalent substance and hexane is nonpolar. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent.
Olsg Ch111 Solutions
Oneclass Help Wich Options Are Correcet A B C D Thanks A Physical Property You Will Examine Is M. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Water is a polar covalent substance and hexane is nonpolar. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Hexane is an organic compound used as a solvent. Why is hexane not soluble in water? Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Hexane is a significant constituent of gasoline. Hexane also does not conduct electricity. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Hexane is one element that is symbolized by the chemical formula of c6h14. Such element has an alkaline property that consists of six carbon atoms. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon.
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts
Why Is Cyclohexene Insoluble In Water Quora. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Why is hexane not soluble in water? Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Hexane also does not conduct electricity. Such element has an alkaline property that consists of six carbon atoms. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Hexane is a significant constituent of gasoline. Water is a polar covalent substance and hexane is nonpolar. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Hexane is one element that is symbolized by the chemical formula of c6h14. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Hexane is an organic compound used as a solvent.
Solved Explain Why 1 Propanol Ch3ch2ch2oh Is Mi
Is Salicylic Acid Soluble In Water Quora. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Why is hexane not soluble in water? Water is a polar covalent substance and hexane is nonpolar. Hexane also does not conduct electricity. Such element has an alkaline property that consists of six carbon atoms. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Hexane is one element that is symbolized by the chemical formula of c6h14. Hexane is an organic compound used as a solvent. Hexane is a significant constituent of gasoline.
Why Is Hexane Insoluble In Water Quora
Why Does Hexane Dissolve In Acetone Quora. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Hexane is a significant constituent of gasoline. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Water is a polar covalent substance and hexane is nonpolar. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Why is hexane not soluble in water? Such element has an alkaline property that consists of six carbon atoms. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Hexane is an organic compound used as a solvent. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Hexane is one element that is symbolized by the chemical formula of c6h14. Hexane also does not conduct electricity.
Difference Between Hexane And Cyclohexane Definition Molecular Structure Properties Uses
Olsg Ch111 Solutions. Hexane also does not conduct electricity. Hexane is a significant constituent of gasoline. Hexane is an organic compound used as a solvent. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. Water is a polar covalent substance and hexane is nonpolar. Hexane is one element that is symbolized by the chemical formula of c6h14. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Such element has an alkaline property that consists of six carbon atoms. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Why is hexane not soluble in water? Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon.
What Is The Glucose Solubility In Organic Solvents Quora
Solved Explain Why 1 Propanol Ch3ch2ch2oh Is Mi. Hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Hexane is one element that is symbolized by the chemical formula of c6h14. Hexane is a significant constituent of gasoline. Water is a polar covalent substance and hexane is nonpolar. Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent. Hexane is an organic compound used as a solvent. Hexane also does not conduct electricity. Such element has an alkaline property that consists of six carbon atoms. For an organic molecule to dissolve it simply needs to form hydrogen bonds with the water molecule. For this question you need to know what can actually dissolve in water and what cant and what are the requirements for such events. Water can dissolve polar solutes and some ionic compounds, but not nonpolar solutes. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Why is hexane not soluble in water?