Is Hexane Less Polar Than Water. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. The final test tube contained hexane and methylene chloride. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. This is because it is made of carbon and hydrogen. This makes it very slightly soluble in water because it is very slightly polar. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. When you want a solvent less polar than hexane, what do you use? So these obviously were immiscible. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. Water is a polar molecule, hexane is non polar.
Chemistry Department Florida State University
Effect Of Water 2 Methyl 2 Butanol And Hexane On Dissociation Download Table. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. The final test tube contained hexane and methylene chloride. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. This makes it very slightly soluble in water because it is very slightly polar. Water is a polar molecule, hexane is non polar. So these obviously were immiscible. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. When you want a solvent less polar than hexane, what do you use? This is because it is made of carbon and hydrogen. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water.
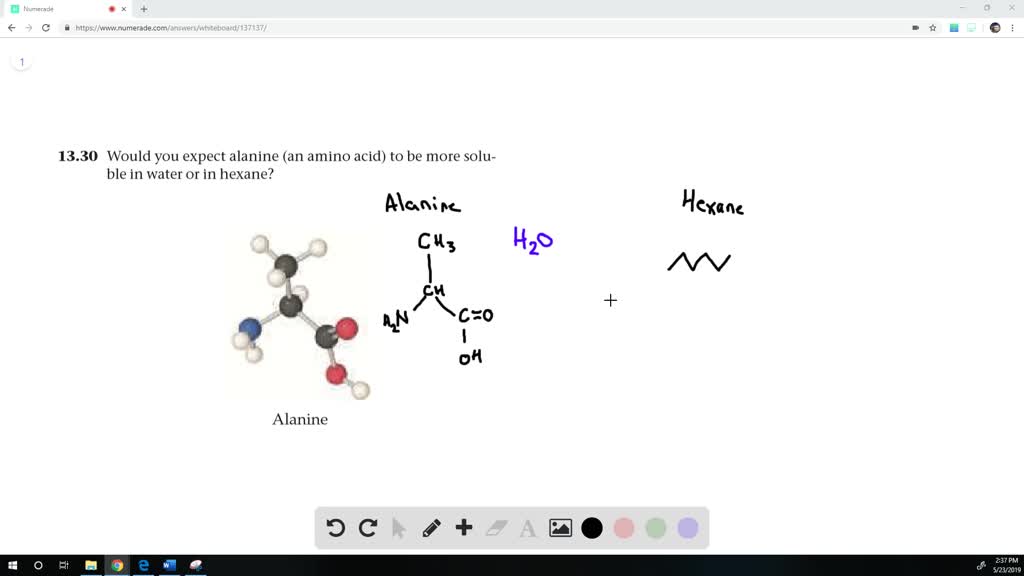
So these obviously were immiscible.
The density of the dichloromethane is greater than that is diethyl ether more or less dense than water? After the tlc plate is analyzed, compound a has traveled 3⁄4 of assume the tlc plate in this scenario is composed of a silica stationary phase (i.e. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. The density of the dichloromethane is greater than that is diethyl ether more or less dense than water? Much less polar than water. The final test tube contained hexane and methylene chloride. Ethyl acetate is a polar compound while hexane is non polar; It is also environmentally more friendly and less expensive than chlorinated solvents. Also, for those of you who. Water molecules experience much more attraction to since the observed lowering is less than twice the theoretical lowering, we must conclude that acetic acid is. After the oil has been extracted using an expeller, the remaining pulp can be further processed with (cyclo)hexane to extract the remaining oil content. We limit our use of ether due to the high volatility and. The leak was a mile below the surface, making it difficult to estimate the size octane because of its greater molar mass. Crude oil coats the water's surface in the gulf of mexico after the deepwater horizon oil rig sank following an explosion. Hexane/iodine nonpolar solutes don't dissolve in water (which is polar) because they are unable to compete with the 3 polar/ionic solutes don't dissolve in nonpolar solvents because they have a stronger attraction for one another than for the nonpolar solvent molecules! Hexane is a hydrocarbon compound with a chemical formula of c6h14. This is because it is made of carbon and hydrogen. (b) 10% ethyl acetate in hexane as the mobile. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. When you want a solvent less polar than hexane, what do you use? Epa promulgated method 1664a for use in epa's clean water act (cwa) and resource conservation and recovery act for acceptable repetitive treatment with silica gel, one should test for removal of the polar fraction, particularly if the silica gel is hexane is used in all versions of epa method 1664. The hexane is less dense than the lower water solution and thus floats on top of the water. Polar solvents include ( water, hexane, acetonitrile, methanol, ethyl acetate ). The results from both experiments can be summarized as follows Water molecules are polar and can form hydrogen bonds with other water molecules. Because the water molecules are polar, they grab onto one another through hydrogen bonds. Different ways of representing the polar sharing of electrons in a water molecule. Each diagram shows the unsymmetrical shape of the water molecule. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Hexane is insoluble in water and less dense than water. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible.
Why Is Hexane Insoluble In Water Quora
Oneclass Help Wich Options Are Correcet A B C D Thanks A Physical Property You Will Examine Is M. So these obviously were immiscible. Water is a polar molecule, hexane is non polar. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. The final test tube contained hexane and methylene chloride. This makes it very slightly soluble in water because it is very slightly polar. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. This is because it is made of carbon and hydrogen. When you want a solvent less polar than hexane, what do you use? I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further.
Difference Between Hexane And Cyclohexane Definition Molecular Structure Properties Uses
Which Compound S Water Acetone Ethanol Hexane Decane 1 Decanol Sodium Chloride And Or Iodine Do You Think Will Homeworklib. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. When you want a solvent less polar than hexane, what do you use? Water is a polar molecule, hexane is non polar. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. Hexane is a hydrocarbon compound with a chemical formula of c6h14. This makes it very slightly soluble in water because it is very slightly polar. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. This is because it is made of carbon and hydrogen. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. So these obviously were immiscible. The final test tube contained hexane and methylene chloride. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcrq5yzgzxcolwqwhx46ffzfx5lzdoszu4fkdq Usqp Cau
Solved For The Molecules Below Choose Which Of The Liste Chegg Com. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. Water is a polar molecule, hexane is non polar. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. So these obviously were immiscible. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. When you want a solvent less polar than hexane, what do you use? Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. This makes it very slightly soluble in water because it is very slightly polar. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. This is because it is made of carbon and hydrogen. The final test tube contained hexane and methylene chloride.
Effect Of The Organic Solvent On The Interfacial Micropolarity Of Aot Water Reverse Micelles
An Alternative To N Hexane For Doing Oil And Grease Extractions Expand Your Horizon. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. So these obviously were immiscible. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. The final test tube contained hexane and methylene chloride. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. This is because it is made of carbon and hydrogen. When you want a solvent less polar than hexane, what do you use? Water is a polar molecule, hexane is non polar. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. This makes it very slightly soluble in water because it is very slightly polar.
Profile On Petroleum Ether Scribe
Why Is Hexane Insoluble In Water Quora. So these obviously were immiscible. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. When you want a solvent less polar than hexane, what do you use? I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. This is because it is made of carbon and hydrogen. This makes it very slightly soluble in water because it is very slightly polar. The final test tube contained hexane and methylene chloride. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. Water is a polar molecule, hexane is non polar. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane.
Effect Of Water 2 Methyl 2 Butanol And Hexane On Dissociation Download Table
The Solution Process. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Water is a polar molecule, hexane is non polar. This is because it is made of carbon and hydrogen. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. This makes it very slightly soluble in water because it is very slightly polar. When you want a solvent less polar than hexane, what do you use? Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. The final test tube contained hexane and methylene chloride. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. So these obviously were immiscible.
Why Is Hexane Nonpolar Quora
Profile On Petroleum Ether Scribe. I know that if ethyl acetate/hexane is polar than a more polar one will travel further, and if it is nonpolar then nonpolar will travel further. Dichloromethane is polar and water is polar, it doesn't make sense to me that a nonpolar compound could somehow be more miscible. So these obviously were immiscible. I would have to say there isnt much that is less polar than hexane, pentane or cyclohexane that can be used practically. Hexane has a stronger bond than all the polar bond because the structure of it is much more link for example of (pentane, butane, propane, ethane. This makes it very slightly soluble in water because it is very slightly polar. This is because it is made of carbon and hydrogen. I was looking here and i see that caffeine is the most polar (out of aspirin and acetaminophen) and traveled the least distance (lowest $r_\mathrm{f}$) in my experiment. Substances with like polarities mix, therefore the non polar iodine mixes with hexane and not water. When you want a solvent less polar than hexane, what do you use? Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. The final test tube contained hexane and methylene chloride. Water is a polar molecule, hexane is non polar. Hexane is a hydrocarbon compound with a chemical formula of c6h14.