Is Hexane Ethyl Acetate Polar Or Nonpolar. As you can see, the image below shows the chemical formula of ethyl acetate. Hence, ethyl acetate is a polar compound. Polar molecule contains bond dipoles, which do not cancel each other. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. I know that the ethyl acetate is polar and the hexane is nonpolar. Ethyl acetate is a polar solvent. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Polar protic vs polar aprotic vs nonpolar: Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Would ethyl acetate/hexane be considered nonpolar or polar overall? Hexane is nonpolar compound as it contains nonpolar carbons. It will be non polar. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds.
A Chromatography Column Packed With Silica Gel As Stationary Phase Was Used To Separate A Mixture Of Compounds Consisting Of A Benzanilide B Aniline And C Acetophenone When The Column Is Eluted
An Overview Of Recent Progress In Solvent Systems Additives And Modifiers Of Counter Current Chromatography New Journal Of Chemistry Rsc Publishing. Hexane is nonpolar compound as it contains nonpolar carbons. Would ethyl acetate/hexane be considered nonpolar or polar overall? Ethyl acetate is a polar solvent. I know that the ethyl acetate is polar and the hexane is nonpolar. Hence, ethyl acetate is a polar compound. It will be non polar. Polar protic vs polar aprotic vs nonpolar: As you can see, the image below shows the chemical formula of ethyl acetate. I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Polar molecule contains bond dipoles, which do not cancel each other. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds.
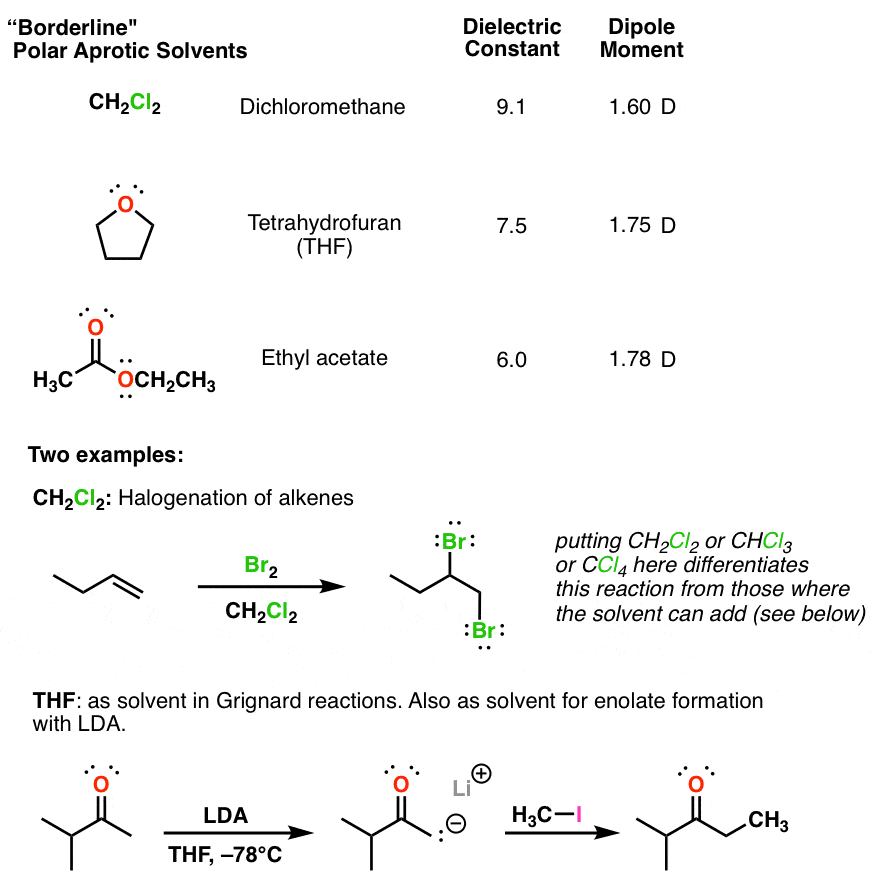
Hexane is nonpolar compound as it contains nonpolar carbons.
Ethyl acetate (systematically ethyl ethanoate, commonly abbreviated etoac, etac or ea) is the organic compound with the formula ch3−coo−ch2−ch3, simplified to c4h8o2. You can substitute with heptane or petroleum ether. Are hexanes non polar or polar? This colorless liquid has a characteristic sweet smell (similar to pear drops) and is used in glues, nail polish removers. The following two solvents systems were found to separate compounds x and y by flash column chromatography: Would ethyl acetate/hexane be considered nonpolar or polar overall? Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). Try to avoid using hexane. As you can see, the image below shows the chemical formula of ethyl acetate. Hexane is less polar than ethyl acetate, so a 100% hexane solvent would be less polar than the 20/80% mix. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Organic chemistry welcome to organic chemistry definition of 'chemistry' and 'organic'. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of bond dipoles may or may not cancel out thereby producing either molecules that are nonpolar, if they cancel, or polar, if they do not cancel. It can be used with a variety of polar solvents. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Polar protic vs polar aprotic vs nonpolar: Its miscibility with water gets higher at elevated temperature. We start with the lewis structure and then. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Benzophenone essentially nonpolar (slightly polar due to the c=o bond) was soluble in methyl. Ethyl acetate is a polar solvent. Ethyl acetate (systematically ethyl ethanoate, commonly abbreviated etoac, etac or ea) is the organic compound with the formula ch3−coo−ch2−ch3, simplified to c4h8o2. Benzophenone, which is largely nonpolar, but possesses a polar carbonyl group, was found to be partially soluble in methyl alcohol and hexane but insoluble in water. Is ethyl acetate polar or non polar. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Polar molecule contains bond dipoles, which do not cancel each other. Because the column is polar, components will not travel straight through but will be slowed down more or less based on their own polarity. About solvents in organic chemistry. Why is ethyl acetate nonpolar? So, if a less polar solvent is pulling, the sample molecules won't be stuck to them as strongly. Is toluene polar or non polar?
Pdf Measurement And Modeling Of Carbon Dioxide Solubility In Polar And Nonpolar Solvent Semantic Scholar
Polar Protic Polar Aprotic Nonpolar All About Solvents. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Polar molecule contains bond dipoles, which do not cancel each other. As you can see, the image below shows the chemical formula of ethyl acetate. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Polar protic vs polar aprotic vs nonpolar: Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. I know that the ethyl acetate is polar and the hexane is nonpolar. Ethyl acetate is a polar solvent. It will be non polar. Hexane is nonpolar compound as it contains nonpolar carbons. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Would ethyl acetate/hexane be considered nonpolar or polar overall? I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Hence, ethyl acetate is a polar compound.
Effect Of Solvent Polarity On Extraction Yield And Antioxidant Properties Of Phytochemicals From Bean Phaseolus Vulgaris Seeds
Principles Of Chromatography Stationary Phase Article Khan Academy. I know that the ethyl acetate is polar and the hexane is nonpolar. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Polar molecule contains bond dipoles, which do not cancel each other. Ethyl acetate is a polar solvent. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Polar protic vs polar aprotic vs nonpolar: Hexane is nonpolar compound as it contains nonpolar carbons. Would ethyl acetate/hexane be considered nonpolar or polar overall? It will be non polar. As you can see, the image below shows the chemical formula of ethyl acetate. Hence, ethyl acetate is a polar compound.
The Weight Of N Hexane Ethyl Acetate And Ethanol Extracts From 100 G Download Scientific Diagram
A Student Developed A Tlc Plate That Was Spotted With A Mixt Clutch Prep. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Ethyl acetate is a polar solvent. I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Polar protic vs polar aprotic vs nonpolar: Would ethyl acetate/hexane be considered nonpolar or polar overall? As you can see, the image below shows the chemical formula of ethyl acetate. Hexane is nonpolar compound as it contains nonpolar carbons. It will be non polar. Polar molecule contains bond dipoles, which do not cancel each other. I know that the ethyl acetate is polar and the hexane is nonpolar. Hence, ethyl acetate is a polar compound. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no.
Doc Question In Chromatogrphy Docx Ehab Aboueladab Academia Edu
The Weight Of N Hexane Ethyl Acetate And Ethanol Extracts From 100 G Download Scientific Diagram. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. As you can see, the image below shows the chemical formula of ethyl acetate. I know that the ethyl acetate is polar and the hexane is nonpolar. Polar protic vs polar aprotic vs nonpolar: Hence, ethyl acetate is a polar compound. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Would ethyl acetate/hexane be considered nonpolar or polar overall? Polar molecule contains bond dipoles, which do not cancel each other. Hexane is nonpolar compound as it contains nonpolar carbons. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Ethyl acetate is a polar solvent. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. It will be non polar.
Effect Of Solvent Polarity On Extraction Yield And Antioxidant Properties Of Phytochemicals From Bean Phaseolus Vulgaris Seeds
Ethyl Acetate Ch3cooc2h5 Pubchem. Would ethyl acetate/hexane be considered nonpolar or polar overall? Hexane is nonpolar compound as it contains nonpolar carbons. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. It will be non polar. I know that the ethyl acetate is polar and the hexane is nonpolar. Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. Polar protic vs polar aprotic vs nonpolar: Polar molecule contains bond dipoles, which do not cancel each other. As you can see, the image below shows the chemical formula of ethyl acetate. Ethyl acetate is a polar solvent. Hence, ethyl acetate is a polar compound. I am trying to understand what if polar or nonpolar analgesics would travel further in tlc.
Experiment 8
2 3d Separation Theory Chemistry Libretexts. I know that the ethyl acetate is polar and the hexane is nonpolar. Hence, ethyl acetate is a polar compound. Polar protic vs polar aprotic vs nonpolar: This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. Would ethyl acetate/hexane be considered nonpolar or polar overall? Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. As you can see, the image below shows the chemical formula of ethyl acetate. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Hexane is nonpolar compound as it contains nonpolar carbons. It will be non polar. Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. Ethyl acetate is a polar solvent. Polar molecule contains bond dipoles, which do not cancel each other.
Dimethyl Sulfoxide High Purity Solvents Sigma Aldrich
Simultaneous Determination Of Active Compounds In Piper Betle Linn Leaf Extract And Effect Of Extracting Solvents On Bioactivity Nguyen 2020 Engineering Reports Wiley Online Library. This compound has two oxygen atoms, whose electronegativity is greater than that of the carbon atom to which they are attached, the dipole moment of this molecule is nonzero. I am trying to understand what if polar or nonpolar analgesics would travel further in tlc. It will be non polar. Hence, ethyl acetate is a polar compound. Polar molecule contains bond dipoles, which do not cancel each other. Values for relative polarity, eluant strength, threshold limits and vapor pressure have been extracted from: Hexane is nonpolar compound as it contains nonpolar carbons. Ethyl acetate is less polar than ethanol, even though it contains more polar bonds. Ethyl acetate is a polar solvent. Would ethyl acetate/hexane be considered nonpolar or polar overall? Because ethyl acetate has both oxygen(2) and hydrogen(8) due to hydrogen bonding the covalent bonds between carbon and hydrogen is slightly shifted towards carbon and oxygen which creates a very little charge on both the sides of ethyl acetate making it much polar than hexane since there is no. As you can see, the image below shows the chemical formula of ethyl acetate. Polar protic vs polar aprotic vs nonpolar: Christian reichardt, solvents and solvent 2 the values for relative polarity are normalized from measurements of solvent shifts of absorption spectra and were extracted from christian. I know that the ethyl acetate is polar and the hexane is nonpolar.