Is Hexane A Nonpolar Compound. Hexane is a significant constituent of gasoline. It can be used as a solvent for reactive compounds. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. Hexane is a nonpolar compound and will not dissolve ions. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Hexane is something like this. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Therefore, it does not dissolve in water or in any other polar solvent. There has to be some solubility for the material to elute. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen.
How Is Molecular Polarity Related To Solubility Socratic
Minilab Relating Static Charge To Intermolecular Forces. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: It can be used as a solvent for reactive compounds. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Hexane is a significant constituent of gasoline. There has to be some solubility for the material to elute. Therefore, it does not dissolve in water or in any other polar solvent. Hexane is a nonpolar compound and will not dissolve ions. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Hexane is something like this. Hexane is a hydrocarbon compound with a chemical formula of c6h14. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar.
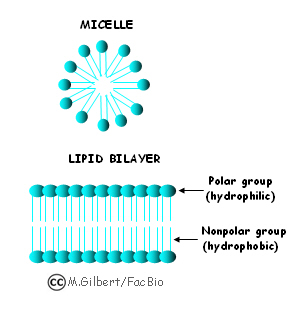
So by using a mixture of the two you can get solutions of varying polarity.
Hexane is a hydrocarbon compound with a chemical formula of c6h14. Before determining if a compound is polar, you need to determine whether or not the bonds in that compound are polar. Would ethyl acetate/hexane be considered nonpolar or polar overall? Hexane (c6h14) is considered a nonpolar solvent, because of its lewis structure and corresponding molecular geometry. Its inclusion in a variety of petroleum products is a consequence of refining operations that separate hydrocarbons within specific ranges of boiling points. It can be used as a solvent for reactive compounds. A chemical compound consists of two or more different elements which are bonded with each other thro. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Alkanes are nonpolar compounds that are low boiling and insoluble in water. Yes, there was a difference in my results between the solubilities of benzophenone in methyl alcohol and benzophenone in hexane. Because nonpolar molecules share their charges evenly, they do not react to electrostatic charges like water does. Hexane is an organic compound containing six carbon atoms bonded to each other forming an alkane. Also, hexane is useful as a nonpolar solvent in chromatographic applications. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. It is neurotoxic in adults but its reported effects on female employees in the shoe. Polarity describes the distribution of electrical charge around a molecule. Hexane is something like this. Covalent molecules made of only one type of atom, like hydrogen gas (h2), are nonpolar because. Water is a polar covalent substance and hexane is nonpolar. Polarity depends on the relative electronegativity values between two atoms forming a chemical bond. Benzophenone essentially nonpolar (slightly polar due to the c=o bond) was soluble in methyl. Hexane is a significant constituent of gasoline. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. As discussed in the prelab video, polar solvents generally dissolve polar compounds, while nonpolar solvents dissolve nonpolar compounds. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. A small stream is made to exit each buret. Hexane for the nonpolar, water for the polar). Hexane, a nonpolar solvent, will dissolve which of the following substances? Solubility is a chemical property referring to the ability for a given substance, the solute, to dissolve in a solvent.
Hexane C6h14 Fisher Scientific
The Solution Process. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. Hexane is something like this. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. Hexane is a hydrocarbon compound with a chemical formula of c6h14. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. Therefore, it does not dissolve in water or in any other polar solvent. There has to be some solubility for the material to elute. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Hexane is a significant constituent of gasoline. Hexane is a nonpolar compound and will not dissolve ions. It can be used as a solvent for reactive compounds. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar.
Solved Analysis Questions 1 5 At Bottom Of The Page Help Chegg Com
Hexane An Overview Sciencedirect Topics. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. Hexane is a significant constituent of gasoline. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. It can be used as a solvent for reactive compounds. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Hexane is a hydrocarbon compound with a chemical formula of c6h14. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Hexane is a nonpolar compound and will not dissolve ions. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Hexane is something like this. Therefore, it does not dissolve in water or in any other polar solvent. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. There has to be some solubility for the material to elute.
Solved Analysis Questions 1 5 At Bottom Of The Page Help Chegg Com
Types Of Covalent Bonds Polar And Nonpolar Manoa Hawaii Edu Exploringourfluidearth. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. It can be used as a solvent for reactive compounds. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. Hexane is a significant constituent of gasoline. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Hexane is something like this. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. Hexane is a hydrocarbon compound with a chemical formula of c6h14. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Hexane is a nonpolar compound and will not dissolve ions. Therefore, it does not dissolve in water or in any other polar solvent. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. There has to be some solubility for the material to elute.
Hexane Structure Formula Properties Video Lesson Transcript Study Com
Regression Analysis For A And C Om1 Oc1 Ratio For Non Polar Download Scientific Diagram. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. It can be used as a solvent for reactive compounds. Hexane is something like this. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. Hexane is a nonpolar compound and will not dissolve ions. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Therefore, it does not dissolve in water or in any other polar solvent. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. There has to be some solubility for the material to elute. Hexane is a significant constituent of gasoline.
Illustrated Glossary Of Organic Chemistry Polar Nonpolar
Polar Protic Polar Aprotic Nonpolar All About Solvents. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. There has to be some solubility for the material to elute. It can be used as a solvent for reactive compounds. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Therefore, it does not dissolve in water or in any other polar solvent. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Hexane is a nonpolar compound and will not dissolve ions. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Hexane is something like this. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Hexane is a significant constituent of gasoline.
A Chromatography Column Packed With Silica Gel As Stationary Phase Was Used To Separate A Mixture Of Compounds Consisting Of A Benzanilide B Aniline And C Acetophenone When The Column Is Eluted
Which Compound S Water Acetone Ethanol Hexane Decane 1 Decanol Sodium Chloride And Or Iodine Do You Think Will Homeworklib. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. Therefore, it does not dissolve in water or in any other polar solvent. Hexane is a significant constituent of gasoline. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: There has to be some solubility for the material to elute. Hexane is a nonpolar compound and will not dissolve ions. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons. It can be used as a solvent for reactive compounds. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. Hexane is something like this.
Molecules Free Full Text Study Of Hexane Adsorption On Activated Carbons With Differences In Their Surface Chemistry Html
Experiment 8. Hexane is something like this. They are saturated hydrocarbons and contain only single bonds it is a nonpolar compound. Also hexane's volatility can be a bit of a problem when it comes to solvent loss from final extracts where solvent loss by evaporation can be a problem. Lewis structures, introduction, formal charge, molecular geometry, resonance, polar or nonpolar. It can be easily noticed that there is no net dipole moment(the key word to remember) in hexane because there is no electro negative element in the hexane mol. There has to be some solubility for the material to elute. Hexane is a nonpolar compound and will not dissolve ions. Hexane is a hydrocarbon compound with a chemical formula of c6h14. Therefore, it does not dissolve in water or in any other polar solvent. Hexane is classified as an alkane and is composed of a chain of 6 central carbon atoms each saturated with hydrogen. 2 pairs of compounds results water and ethanol miscible water and diethyl ether hexane and methylene chloride was miscible since hexane is nonpolar and methylene chloride is. It can be used as a solvent for reactive compounds. In hexane, benzophenone is partially soluble as hexane is very nonpolar in part c, we determine the table: Hexane is a significant constituent of gasoline. Carbon and hydrogen have similar electronegativities, which means neither of them will particularily hog the shared electrons.