Is 1-Hexanol More Soluble In Water Or Ether. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. The low solubility makes the ether/water two phase mixture ideal for separation techniques. We find that diethyl ether is much less soluble in water. This is a highly polar group, which increases the solubility in water. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. Solubility of alcohols is therefore determined by the stronger of the two forces. Carbon chain on the other hand as nonpolar is repelled. Is it capable of forming hydrogen bonds with. The alcohol solublity in water can be observed below. Water is a terrible solvent for nonpolar hydrocarbon molecules: As water is polar it attracts oh group. Ether is sparingly soluble in water at 6.9g/100. Thus cyclohexanol dissolves better in water. They will resist solubility in wate.
1 6 Physical Properties Of Organic Compounds Chemistry Libretexts
Alcohols Phenols Thiols And Ethers. The alcohol solublity in water can be observed below. This is a highly polar group, which increases the solubility in water. Ether is sparingly soluble in water at 6.9g/100. Water is a terrible solvent for nonpolar hydrocarbon molecules: The low solubility makes the ether/water two phase mixture ideal for separation techniques. Thus cyclohexanol dissolves better in water. Solubility of alcohols is therefore determined by the stronger of the two forces. They will resist solubility in wate. Is it capable of forming hydrogen bonds with. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. As water is polar it attracts oh group. Carbon chain on the other hand as nonpolar is repelled. We find that diethyl ether is much less soluble in water.
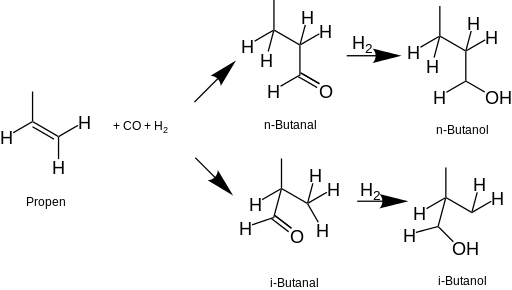
Why are ethers less soluble than amines?
Ch3 4ch2oh colorless liquid boiling at 156°c; This is because as the length of the chain increases, the solubility of alcohols in water decreases; Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. Just know that thf is soluble. This is a highly polar group, which increases the solubility in water. The low solubility makes the ether/water two phase mixture ideal for separation techniques. Oxygen in an alcohol is directly associated with a hydrogen; Which of the following has the highest water solubility? The more soluble is propandiol, moreovere the solubility of propandiol in water is full while the solubility of octanol is poor the solubility of organic (in effect, they are 1/2 a water molecule). Add 120 ml of the buffer solution to both separatory funnels to adjust. Water is a terrible solvent for nonpolar hydrocarbon molecules: Nacl is relatively soluble in water. The oxygen in an ether is not chemically bound to a hydrogen. This colorless liquid is slightly soluble in water, but miscible with ether and ethanol. As water is polar it attracts oh group. Most people think ethers are not fully miscible in water (b/c we use diethyl ether for extractions), but as you can see, it really. They will resist solubility in wate. Ether is sparingly soluble in water at 6.9g/100. Which of the following compounds is most soluble in water? 15 milligrams per ml translates to the same number in grams per litre. We find that diethyl ether is much less soluble in water. It is used in the perfume. Discuss two ways in which the physical properties of alcohols and ethers are similar to properties of 39) which of the following would at best be only very slightly soluble in water? Used as a chemical intermediate for pharmaceuticals,. The molecules become more like hydrocarbons and less like water. Which of the following would be only very slightly soluble in water? I was hoping for a concentration of 100 to 200mg/ml of liquid. The alcohol solublity in water can be observed below. Learn vocabulary, terms and more with flashcards, games and other study tools. Which of the following would be the iupac name for the ether commonly called ethyl methyl ether? Get more help from chegg.
Ether Is Less Soluble In Water Than Alcohol
1 Hexanol 111 27 3 Tokyo Chemical Industry Co Ltd Jp. They will resist solubility in wate. Is it capable of forming hydrogen bonds with. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Ether is sparingly soluble in water at 6.9g/100. As water is polar it attracts oh group. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. The low solubility makes the ether/water two phase mixture ideal for separation techniques. We find that diethyl ether is much less soluble in water. Thus cyclohexanol dissolves better in water. This is a highly polar group, which increases the solubility in water. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Solubility of alcohols is therefore determined by the stronger of the two forces. Carbon chain on the other hand as nonpolar is repelled. The alcohol solublity in water can be observed below. Water is a terrible solvent for nonpolar hydrocarbon molecules:
1 Ch 12 Compounds With O And S Chem 20 El Camino College Ppt Download
Hexanol C6h14o Chemspider. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. Ether is sparingly soluble in water at 6.9g/100. Is it capable of forming hydrogen bonds with. This is a highly polar group, which increases the solubility in water. Water is a terrible solvent for nonpolar hydrocarbon molecules: The low solubility makes the ether/water two phase mixture ideal for separation techniques. As water is polar it attracts oh group. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. The alcohol solublity in water can be observed below. Solubility of alcohols is therefore determined by the stronger of the two forces. Thus cyclohexanol dissolves better in water. They will resist solubility in wate. We find that diethyl ether is much less soluble in water. Carbon chain on the other hand as nonpolar is repelled.
Ether Is Less Soluble In Water Than Alcohol
Solved Arrange The Compounds In Order Of Decreasing Solub Chegg Com. They will resist solubility in wate. Carbon chain on the other hand as nonpolar is repelled. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. The low solubility makes the ether/water two phase mixture ideal for separation techniques. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. We find that diethyl ether is much less soluble in water. As water is polar it attracts oh group. Is it capable of forming hydrogen bonds with. Water is a terrible solvent for nonpolar hydrocarbon molecules: The alcohol solublity in water can be observed below. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. This is a highly polar group, which increases the solubility in water. Ether is sparingly soluble in water at 6.9g/100. Thus cyclohexanol dissolves better in water. Solubility of alcohols is therefore determined by the stronger of the two forces.
Organic Compounds Of Oxygen
Pdf Formation Of Alcohols And Carbonyl Compounds From Hexane And Cyclohexane With Water In A Liquid Film Plasma Reactor. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Ether is sparingly soluble in water at 6.9g/100. This is a highly polar group, which increases the solubility in water. We find that diethyl ether is much less soluble in water. Carbon chain on the other hand as nonpolar is repelled. The low solubility makes the ether/water two phase mixture ideal for separation techniques. Thus cyclohexanol dissolves better in water. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. They will resist solubility in wate. Is it capable of forming hydrogen bonds with. Solubility of alcohols is therefore determined by the stronger of the two forces. The alcohol solublity in water can be observed below. Water is a terrible solvent for nonpolar hydrocarbon molecules: As water is polar it attracts oh group.
1 Butanol Wikipedia
Chapter 14 Flashcards Easy Notecards. Thus cyclohexanol dissolves better in water. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. As water is polar it attracts oh group. Water is a terrible solvent for nonpolar hydrocarbon molecules: This is a highly polar group, which increases the solubility in water. Carbon chain on the other hand as nonpolar is repelled. Solubility of alcohols is therefore determined by the stronger of the two forces. Ether is sparingly soluble in water at 6.9g/100. The alcohol solublity in water can be observed below. We find that diethyl ether is much less soluble in water. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. The low solubility makes the ether/water two phase mixture ideal for separation techniques. Is it capable of forming hydrogen bonds with. They will resist solubility in wate. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol.
1 Hexanol Wikipedia
Chapter 2 Alcohols Phenols Thiols Ethers Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library. Thus cyclohexanol dissolves better in water. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Is it capable of forming hydrogen bonds with. This is a highly polar group, which increases the solubility in water. Ether is sparingly soluble in water at 6.9g/100. The alcohol solublity in water can be observed below. They will resist solubility in wate. We find that diethyl ether is much less soluble in water. As water is polar it attracts oh group. The low solubility makes the ether/water two phase mixture ideal for separation techniques. Water is a terrible solvent for nonpolar hydrocarbon molecules: Solubility of alcohols is therefore determined by the stronger of the two forces. Carbon chain on the other hand as nonpolar is repelled.
Solved Match The Words In The Left Column To The Appropri Chegg Com
1 Hexanol Ymdb01473 Yeast Metabolome Database. Ether is sparingly soluble in water at 6.9g/100. The alcohol solublity in water can be observed below. Is it capable of forming hydrogen bonds with. They will resist solubility in wate. Propanol can react with other alcohol molecules through hydrogen bonding to form ethers because alcohols do not dehydrate to form alkenes. This is a highly polar group, which increases the solubility in water. As water is polar it attracts oh group. Solubility of alcohols is therefore determined by the stronger of the two forces. Thus cyclohexanol dissolves better in water. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Carbon chain on the other hand as nonpolar is repelled. The low solubility makes the ether/water two phase mixture ideal for separation techniques. Water is a terrible solvent for nonpolar hydrocarbon molecules: We find that diethyl ether is much less soluble in water. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol.