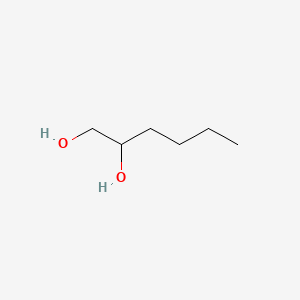
Hexylene Glycol Polar Or Nonpolar. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. We start with the lewis structure and then. However due to the stabilization from intramolecular hydrogen bond. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). The resulting covalent bond is called a polar covalent bond. Why does that rule about dipoles cancelling out not apply to ethylene glycol? Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. Are more easily to be solved in diesel. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. For example, water is 80, ethylene glycol 37, and ethanol 24.3. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers.
Ethylene Glycol Molecule Of The Month June 2018 Html Version
1987 Agrapidis Paloympis And Nash The Effect Of Solvents On The Ultraviolet Absorbance Of Sunscreens Ultraviolet Solvent. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Are more easily to be solved in diesel. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. Why does that rule about dipoles cancelling out not apply to ethylene glycol? Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. The resulting covalent bond is called a polar covalent bond. However due to the stabilization from intramolecular hydrogen bond. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. We start with the lewis structure and then. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). For example, water is 80, ethylene glycol 37, and ethanol 24.3. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule.
Ethylene glycol is the most common antifreeze fluid for standard heating and cooling applications.
Polar protic vs polar aprotic vs nonpolar: About solvents in organic chemistry. Ethylene glycol, hoch2ch2oh, may look nonpolar when drawn, but an internal hydrogen bond results in an electric dipole moment. Polar bonds have high melting point, surface tension, boiling point and low vapour pressure. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. Questions you may have include C2h6o2 ( ethylene glycol ). These were some important difference between nonpolar and polar. They are part of the formulation of paints the ethylene glycol molecule is larger and less polar than water. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). I'll tell you the polar or nonpolar list below. Potassium permanganate ch2s nitrogen monoxide ch3cho glass sh coh2 sof4 dna ch3ch2nh2 malonic acid ethylene glycol isopropyl alcohol hydrogen bromide secl4 hydrogen peroxide ozone c3h6o. Different atoms show attraction to electrons in various degrees. Why does that rule about dipoles cancelling out not apply to ethylene glycol? We start with the lewis structure and then. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. Atoms of different or same elements come together to form molecules. Get examples of polar and nonpolar molecules. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. Which compound do you expect to be miscible with octane (c8h18)? Polar protic vs polar aprotic vs nonpolar: However due to the stabilization from intramolecular hydrogen bond. A lot of students i talk to have questions about solvents, so i've decided to put together a reference post on them. Hydrocarbon liquids, such as gasoline. Learn whether a molecule with polar bonds can be nonpolar. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. If you want to quickly find the word you want to search, use ctrl + f, then type the word you want to search. Initially, patients may be asymptomatic, but ethylene glycol is rapidly absorbed. Here's some real world experience: Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar.
Is Ethylene Glycol Polar Or Nonpolar
Hexylene Glycol. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). We start with the lewis structure and then. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. The resulting covalent bond is called a polar covalent bond. Are more easily to be solved in diesel. For example, water is 80, ethylene glycol 37, and ethanol 24.3. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. Why does that rule about dipoles cancelling out not apply to ethylene glycol? Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. However due to the stabilization from intramolecular hydrogen bond. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low.
Synthesis Characterization And Thermal Properties Of New Flavor Compounds Springerlink
De69628897t2 Waterproof Ink Composition For Printers Google Patents. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. For example, water is 80, ethylene glycol 37, and ethanol 24.3. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. Are more easily to be solved in diesel. We start with the lewis structure and then. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. Why does that rule about dipoles cancelling out not apply to ethylene glycol? However due to the stabilization from intramolecular hydrogen bond. Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. The resulting covalent bond is called a polar covalent bond. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule.
Us 9682021 B2 Substantially Non Aqueous Foamable Petrolatum Based Pharmaceutical And Cosmetic Compositions And Their Uses The Lens Free Open Patent And Scholarly Search
Chemical Compound 99 5 Formamide Liquid Wholesaler From Mumbai. Are more easily to be solved in diesel. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. Why does that rule about dipoles cancelling out not apply to ethylene glycol? However due to the stabilization from intramolecular hydrogen bond. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. We start with the lewis structure and then. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. The resulting covalent bond is called a polar covalent bond. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. For example, water is 80, ethylene glycol 37, and ethanol 24.3. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists.
Pdf An Overview On 2 Methyl 2 4 Pentanediol In Crystallization And In Crystals Of Biological Macromolecules
Dielectric Constant Correlations With Solubility And Solubility Parameters. That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. We start with the lewis structure and then. However due to the stabilization from intramolecular hydrogen bond. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. Are more easily to be solved in diesel. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. Why does that rule about dipoles cancelling out not apply to ethylene glycol? Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. For example, water is 80, ethylene glycol 37, and ethanol 24.3. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). The resulting covalent bond is called a polar covalent bond. Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low.
Synthesis Characterization And Thermal Properties Of New Flavor Compounds Springerlink
Dielctric Constant And Solubility. The resulting covalent bond is called a polar covalent bond. For example, water is 80, ethylene glycol 37, and ethanol 24.3. Are more easily to be solved in diesel. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists. We start with the lewis structure and then. That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Why does that rule about dipoles cancelling out not apply to ethylene glycol? Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. However due to the stabilization from intramolecular hydrogen bond. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule.
Pdf Reaction Of Niobium Pentaethoxide With Glycols
Solved Question 7 Based On The Like Dissolves Like Prin Chegg Com. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. For example, water is 80, ethylene glycol 37, and ethanol 24.3. That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). Why does that rule about dipoles cancelling out not apply to ethylene glycol? However due to the stabilization from intramolecular hydrogen bond. The resulting covalent bond is called a polar covalent bond. Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. We start with the lewis structure and then. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. Are more easily to be solved in diesel. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists.
Investigation On Molecular Interaction Of Glycols In Methanol Solutions Of Methylparaben Methyl 4 Hydroxybenzoate At Different Temperatures Through Thermo Acoustical Analysis Sciencedirect
Synthesis Characterization And Thermal Properties Of New Flavor Compounds Springerlink. Ethylene glycol and its oligomers are preferable as a starting material instead of water, because they allow the creation of polymers with a low. Another way to think about it is that ethanol has one alcohol group, and ethylene glycol has two, so it is more polar. For example, water is 80, ethylene glycol 37, and ethanol 24.3. The polarity of molecule could be decided by its dipole moment, a larger dipole moment means a higher polarity of the molecule. However due to the stabilization from intramolecular hydrogen bond. Polyethylene glycol is also commonly used as a polar stationary phase for gas chromatography, as well as a heat transfer fluid in electronic testers. We start with the lewis structure and then. I am reading online that ethylene glycol is polar and ch2cl is, which is confusing me. Learn to determine if c2h4 is polar or nonpolar based on the lewis structure and the molecular geometry (shape). Are more easily to be solved in diesel. Why does that rule about dipoles cancelling out not apply to ethylene glycol? Strictly speaking, if ethylene glycol is drawn in the trans configuration, it is nonpolar. That is why gasoline will mix with it (nonpolar) and water will mix with it (polar). The resulting covalent bond is called a polar covalent bond. Ethylene glycol, hydrogenation and glycols | researchgate, the professional network for scientists.