Hexane Polar Or Nonpolar Molecule. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. A dipole forms, with part of the molecule carrying a. How to determine polarity in a molecule. Atoms of different or same elements come together to form molecules. Polar and nonpolar molecules are the two broad classes of molecules. Polarity describes the distribution of electrical charge around a molecule. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. This is because it is made of carbon and hydrogen. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. Examples include pentane, hexane, and carbon dioxide. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. What's more, hexane has a very symmetrical. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other.
Polarity Activities
Solved 2 You Want To Separate A Polar And Non Polar Comp Chegg Com. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. How to determine polarity in a molecule. Examples include pentane, hexane, and carbon dioxide. What's more, hexane has a very symmetrical. Atoms of different or same elements come together to form molecules. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. This is because it is made of carbon and hydrogen. Polar and nonpolar molecules are the two broad classes of molecules. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. Polarity describes the distribution of electrical charge around a molecule. A dipole forms, with part of the molecule carrying a. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes.
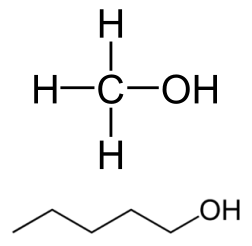
Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5.
Molecules with polar covalent bonds have a positive and negative side. Her's my answer pls check. A structure in which electron density is more or less evenly distributed is called nonpolar. Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded. Although the intramolecular bonds are polar, the molecule is nonpolar. This can be achieved by the molecule having Because both of these molecules are nonpolar, they both did not form hydrogen bonds, and the solution was miscible. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. First, at least one covalent bond in the molecule has to be a polar covalent bond. A nonpolar molecule means that the distribution of charge within the molecule is uniform throughout (like hexane). Polar compounds also tend to have the ability to form hydrogen bonds with water, which would be very. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. About solvents in organic chemistry. Occasionally, randomly, the electron density in a molecule will shift to produce a spontaneous dipole: A completely polar bond occurs when one of the atoms is so electronegative that it takes an electron. The difference between polar and nonpolar bonds stems from the difference in electronegativity of the atoms involved in the bond. The final test tube contained hexane and methylene chloride. Which of these is an amphiphilic molecule? This would lead us to further hypothesize that water has a high melting point (mp) and boiling point (bp) than co2. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. What two conditions necessary for molecules to be polar? Titrate nonpolar and polar substances. 3:1:1 hexane/acetone/acetic acid would be considered quite a polar solvent mix. This will occur among atoms that have similar electronegativity. A dipole forms, with part of the molecule carrying a. Water is a polar molecule with positive charges on one side and negative on the other. How to determine polarity in a molecule. Polar molecules interact with other molecules of similar polarity to form solutions. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. Polar vs nonpolar, what's the difference and how do i remember which is which? When the electronegativities are not equal, electrons are not shared equally and partial ionic charges.
De69530020t2 Organic Matter Extraction Method Solvent To Be Used In This Method And Method Of Measuring The Organic Matter Content Google Patents
Pdf Measurement And Modeling Of Carbon Dioxide Solubility In Polar And Nonpolar Solvent Semantic Scholar. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. Examples include pentane, hexane, and carbon dioxide. How to determine polarity in a molecule. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Polar and nonpolar molecules are the two broad classes of molecules. Atoms of different or same elements come together to form molecules. Polarity describes the distribution of electrical charge around a molecule. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. This is because it is made of carbon and hydrogen. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. A dipole forms, with part of the molecule carrying a. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. What's more, hexane has a very symmetrical.
Journal Of Agricultural Biotechnology And Sustainable Development The Influence Of Solvent S Polarity On Physicochemical Properties And Oil Yield Extracted From Pumpkin Cucurbita Maxima Seed
Solved Sketch Indicate Polar Bonds Lewis Structure N H Chegg Com. Polar and nonpolar molecules are the two broad classes of molecules. What's more, hexane has a very symmetrical. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. A dipole forms, with part of the molecule carrying a. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. Examples include pentane, hexane, and carbon dioxide. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. This is because it is made of carbon and hydrogen. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. How to determine polarity in a molecule. Polarity describes the distribution of electrical charge around a molecule. Atoms of different or same elements come together to form molecules.
Polarity Activities
Hexane Market Revenue Worth Us 2 5 Bn By 2027. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. Polarity describes the distribution of electrical charge around a molecule. What's more, hexane has a very symmetrical. Polar and nonpolar molecules are the two broad classes of molecules. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. This is because it is made of carbon and hydrogen. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Atoms of different or same elements come together to form molecules. How to determine polarity in a molecule. A dipole forms, with part of the molecule carrying a. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. Examples include pentane, hexane, and carbon dioxide.
Hexane An Overview Sciencedirect Topics
Oneclass Explain The Types Of Intermolecular Forces On Hexane. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. Examples include pentane, hexane, and carbon dioxide. Atoms of different or same elements come together to form molecules. This is because it is made of carbon and hydrogen. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. What's more, hexane has a very symmetrical. How to determine polarity in a molecule. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. Polarity describes the distribution of electrical charge around a molecule. A dipole forms, with part of the molecule carrying a. Polar and nonpolar molecules are the two broad classes of molecules.
Explain Why The Ions Na And Mathrm Cl Are
Preparation Of Medicinal Plants Basic Extraction And Fractionation Procedures For Experimental Purposes Abubakar Ar Haque M J Pharm Bioall Sci. A dipole forms, with part of the molecule carrying a. Polar and nonpolar molecules are the two broad classes of molecules. This is because it is made of carbon and hydrogen. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. Polarity describes the distribution of electrical charge around a molecule. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. How to determine polarity in a molecule. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. Examples include pentane, hexane, and carbon dioxide. Atoms of different or same elements come together to form molecules. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. What's more, hexane has a very symmetrical. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges.
Sorption Of Non Polar Molecules On The Gole Sepiolite A Cyclohexane Download Scientific Diagram
The Solution Process. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Examples include pentane, hexane, and carbon dioxide. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. This is because it is made of carbon and hydrogen. A dipole forms, with part of the molecule carrying a. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other. Polar and nonpolar molecules are the two broad classes of molecules. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. Polarity describes the distribution of electrical charge around a molecule. How to determine polarity in a molecule. What's more, hexane has a very symmetrical. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. Atoms of different or same elements come together to form molecules.
Solved Sketch Indicate Polar Bonds Lewis Structure N H Chegg Com
Solved Analysis Questions 1 5 At Bottom Of The Page Help Chegg Com. Here's a look at what polar and nonpolar polar molecules occur when two atoms do not share electrons equally in a covalent bond. When the electronegativities are not equal, electrons are not shared equally and partial ionic charges. This is because it is made of carbon and hydrogen. Although there is a difference in their electronegativity on pauling scale but it is less than 0.5( it is 0.4 approximately) and for significant dipole moment the electronegativity difference should be more than or equal to 0.5. Polarity describes the distribution of electrical charge around a molecule. Examples include pentane, hexane, and carbon dioxide. The bond which is formed by sharing a pair of electrons between two atoms is called a covalent bond. Atoms of different or same elements come together to form molecules. Some molecules are clearly polar or nonpolar, while others fall somewhere on the spectrum between two classes. What's more, hexane has a very symmetrical. How to determine polarity in a molecule. Polar and nonpolar molecules are the two broad classes of molecules. Here are examples of polar and nonpolar molecules, a look at how polarity relates to ionic and covalent bonds, and how you can use polarity. A dipole forms, with part of the molecule carrying a. Depending on the relative electronegativities of the two atoms sharing electrons, there may be partial transfer of electron density from one atom to the other.