Give Reason Hexanol Is Less Soluble In Water Than Butanol. There are some reasons which will decide solubility of organic compounds in water. This is why hexanol is not soluble in water. Solubility of alcohols is therefore determined by the stronger of the two forces. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. They will resist solubility in wate. The alcohol solublity in water can be observed below. Carbon chain on the other hand as nonpolar is repelled. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. This is a highly polar group, which increases the solubility in water. As water is polar it attracts oh group. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. It is soluble in water (77 g/l). When hydrocarbon part of molecule becomes simple or small, it also increases the solubility.
Experiment 4 Purification Recrystallization Of Benzoic Acid
21 9 Alcohols Chemistry Libretexts. They will resist solubility in wate. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. As water is polar it attracts oh group. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. There are some reasons which will decide solubility of organic compounds in water. The alcohol solublity in water can be observed below. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. Solubility of alcohols is therefore determined by the stronger of the two forces. It is soluble in water (77 g/l). This is why hexanol is not soluble in water. Carbon chain on the other hand as nonpolar is repelled. This is a highly polar group, which increases the solubility in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol.
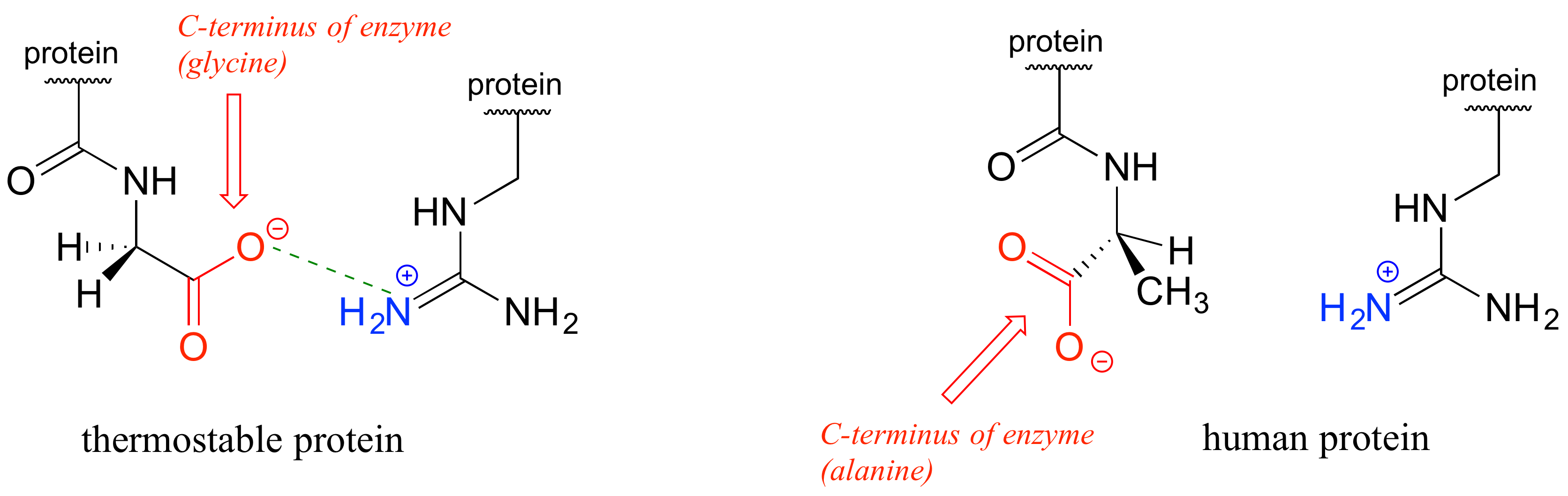
So because hexanol has 6 carbons and ethanol only has 2, hexanol has the lowest solubility.
So larger alcohols like pentanol, hexanol, heptanol and octanol become insoluble in water. The primary reason for the decline in the popularity of soap is the reaction between soap and. Both a and b d. Benzaldehyde and cyclohexanone are both not very soluble in water because of their relatively large carbon chains cyclohexanone is slightly more soluble though, probably because it has one less carbon atom in the carbon chain so. Water solubility of particles seems to play a role in the activation and growth with butanol vapor in the cpc (condensation particle counter) independently figure 7 gives the measured detection efficiency of the cpc for positive ions (monomer and dimer) of teabr. So because hexanol has 6 carbons and ethanol only has 2, hexanol has the lowest solubility. Used as a chemical intermediate for pharmaceuticals,. Despite these few limitations, water's ability do dissolve ionic compounds is one of the major reasons it is so vital to life on earth. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. Today soap represents less than 20% of the market for cleaning agents. The helium is less soluble in the bodily fluids and so less dissolves for a given pressure. Ch3 4ch2oh colorless liquid boiling at 156°c; Which of the following has the highest water solubility? This is why hexanol is not soluble in water. Solubility of alcohols is therefore determined by the stronger of the two forces. Sulfur is less electronegative than oxygen c. As the solvent becomes more nonpolar, the solubility of this polar solute decreases. Nacl is relatively soluble in water. The write the equations involved. Not soluble i think, for the same reason as benzaldehyde. Give a reason for your answer. This is a highly polar group, which increases the solubility in water. It'd still be a little bit soluble. As water is polar it attracts oh group. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. They will resist solubility in wate. This is because as the length of the chain increases, the solubility of alcohols in water decreases; 1.(a) hexanol will be more soluble as compared to the octanol because as the view the full. Alcohols are soluble in water due to hydrogen bonding. Especially smaller chained alcohols are soluble in water. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol.
Green Solvent Processable Strategies For Achieving Large Scale Manufacture Of Organic Photovoltaics Journal Of Materials Chemistry A Rsc Publishing
Physical Properties Of Alcohols. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. They will resist solubility in wate. It is soluble in water (77 g/l). When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. This is a highly polar group, which increases the solubility in water. The alcohol solublity in water can be observed below. There are some reasons which will decide solubility of organic compounds in water. Carbon chain on the other hand as nonpolar is repelled. This is why hexanol is not soluble in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. Solubility of alcohols is therefore determined by the stronger of the two forces. As water is polar it attracts oh group.
Alcohols 1 Nomenclature And Properties Master Organic Chemistry
Ch105 Chapter 9 Organic Compounds Of Oxygen Chemistry. The alcohol solublity in water can be observed below. This is a highly polar group, which increases the solubility in water. This is why hexanol is not soluble in water. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. They will resist solubility in wate. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. There are some reasons which will decide solubility of organic compounds in water. Carbon chain on the other hand as nonpolar is repelled. Solubility of alcohols is therefore determined by the stronger of the two forces. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. As water is polar it attracts oh group. It is soluble in water (77 g/l). At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar.
14 3 Physical Properties Of Alcohols Chemistry Libretexts
Experiment 4 Purification Recrystallization Of Benzoic Acid. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. There are some reasons which will decide solubility of organic compounds in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. This is why hexanol is not soluble in water. Carbon chain on the other hand as nonpolar is repelled. As water is polar it attracts oh group. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. The alcohol solublity in water can be observed below. They will resist solubility in wate. This is a highly polar group, which increases the solubility in water. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. Solubility of alcohols is therefore determined by the stronger of the two forces. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. It is soluble in water (77 g/l).
Why Are Some Combinations Of Liquids Miscible But Others Not Why Is Ethanol Soluble In Water While Ethane Is Insoluble And Butanol Is Less Soluble Quora
Solubility And Molecular Structure. As water is polar it attracts oh group. This is a highly polar group, which increases the solubility in water. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. There are some reasons which will decide solubility of organic compounds in water. They will resist solubility in wate. Solubility of alcohols is therefore determined by the stronger of the two forces. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. Carbon chain on the other hand as nonpolar is repelled. This is why hexanol is not soluble in water. The alcohol solublity in water can be observed below. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. It is soluble in water (77 g/l).
Aqueous Solubility Tests
Chapter 2 Alcohols Phenols Thiols Ethers Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. Carbon chain on the other hand as nonpolar is repelled. This is a highly polar group, which increases the solubility in water. As water is polar it attracts oh group. The alcohol solublity in water can be observed below. This is why hexanol is not soluble in water. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. Solubility of alcohols is therefore determined by the stronger of the two forces. It is soluble in water (77 g/l). As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. There are some reasons which will decide solubility of organic compounds in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. They will resist solubility in wate.
Why Hexanol Is Less Soluble In Water Than Butanol Brainly In
4 4 Solubility Chemistry Libretexts. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. As water is polar it attracts oh group. Solubility of alcohols is therefore determined by the stronger of the two forces. This is why hexanol is not soluble in water. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. It is soluble in water (77 g/l). Carbon chain on the other hand as nonpolar is repelled. Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. They will resist solubility in wate. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. The alcohol solublity in water can be observed below. This is a highly polar group, which increases the solubility in water. There are some reasons which will decide solubility of organic compounds in water.
Chapter 2 Alcohols Phenols Thiols Ethers Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library
4 4 Solubility Chemistry Libretexts. As the length of the carbon chain increases, the alcohol would become increasingly insoluble in yes, as it is rather unpolar. As water is polar it attracts oh group. When hydrocarbon part of molecule becomes simple or small, it also increases the solubility. Carbon chain on the other hand as nonpolar is repelled. This is why hexanol is not soluble in water. They will resist solubility in wate. Q.complete the following reactions bcoz butanol contain more hydroxyl ions (oh) which make bond with h+ ion whereas hexanol contain less ion thats why it is less soluble in water. At some point in the homologous series, the interactions of the hydrophobic chain become greater than the hydrophilic interactions of the alcohol. There are some reasons which will decide solubility of organic compounds in water. The alcohol solublity in water can be observed below. It is soluble in water (77 g/l). Butanol has a short enough aliphatic chain that it is still somewhat soluble in water. Solubility of alcohols is therefore determined by the stronger of the two forces. This is a highly polar group, which increases the solubility in water. If you mean how soluble they are in water, then the order is ethanol< butanol< hexanol.