Explain In Terms Of Molecular Polarity Why Hexane Is Nearly Insoluble In Water. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). Study percent by mass, volume. Polarity is quintessential when it comes to solubility: Hexane also does not conduct. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. Toluene is not a polar molecular it cannot dissolve in water which is polar. Water molecules have true electrical dipoles. However water and hexanes don't have anything in common. What makes hexane an interesting hydrocarbon compound is its insolubility in water. This further contributes the ability of hydrocarbon to become water insoluble. This is where the term polar comes from: (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. Explain why nacl dissolves in water.
Solved 5 Sketch Expanded Condensed And Line Angle Stru Chegg Com
Entropy And Polarity Control The Partition And Transportation Of Drug Like Molecules In Biological Membrane Scientific Reports. Water molecules have true electrical dipoles. Explain why nacl dissolves in water. Polarity is quintessential when it comes to solubility: Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. However water and hexanes don't have anything in common. Study percent by mass, volume. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. Hexane also does not conduct. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. This further contributes the ability of hydrocarbon to become water insoluble. Toluene is not a polar molecular it cannot dissolve in water which is polar. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. What makes hexane an interesting hydrocarbon compound is its insolubility in water. This is where the term polar comes from: There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular).
This continuum can be expressed in terms of resonance by regarding a bond between atoms a and b as in contrast, the water molecule is polar.
Benzoic acid forms a white precipitate in the water. The hydrogen bonding that occurs in water leads to some unusual, but very important properties. The polarity of a bond is the distribution of electrical charge over the atoms joined by the bond. Water molecules attract or are attracted to other polar molecules. It is more difficult to predict the solubility of polar molecular substances than to predict the solubility of ionic compounds. Nacl or table salt is a ionic molecule that has one cation from the sodium and one anion from the chloride. Describe how molecular geometry plays a role in determining whether a molecule is polar or nonpolar. This continuum can be expressed in terms of resonance by regarding a bond between atoms a and b as in contrast, the water molecule is polar. This further contributes the ability of hydrocarbon to become water insoluble. Base your answers to questions 81 and 82 on the information below and on your knowledge of chemistry. Molecules that do not dissolve in water key terms. The hydrogen atom attracts electrons much more strongly than the oxygen atom. Molecules are groups of atoms bonded together. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. It however can also be dissolved in water because of the polar carbonyl region. You can determine the polarity of a molecule by analyzing its bonds, testing how it interacts. Water has a polar covalent molecule. What makes hexane an interesting hydrocarbon compound is its insolubility in water. Amine which have less molecular mass are soluble in water. Gasoline is a mixture of hydrocarbons, including hexane. Why is benzoic acid, glucose, and sodium sulfate insoluble in acetonitrile? Explain why water is polar. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. Remember that even though the covalent bond between each hydrogen and oxygen in water is polar, a water molecule is an electrically neutral molecule overall. This video looks at how to determine polarity in a molecule by understanding how the bond polarities, molecule shape, and outside atoms influence polarity. Benzoic acid forms a white precipitate in the water. Most ionic compounds are soluble in water because the electrostatic forces of the polar water molecules are stronger than the electrostatic forces in addition, the hydrogen molecules are bonded at an angle less than 180 degrees from one another, making water molecules highly polar, with the. Nonpolar compounds don't dissolve well in water (like how oil, a nonpolar solution, forms beads in water). Hexane also does not conduct. We find an explanation of the fact in the atomic theory of crystals, the theory that in crystals the atoms are in a regular order. The bond of nacl when added to water dissolves in water becoming free from the nacl bond becoming polar.
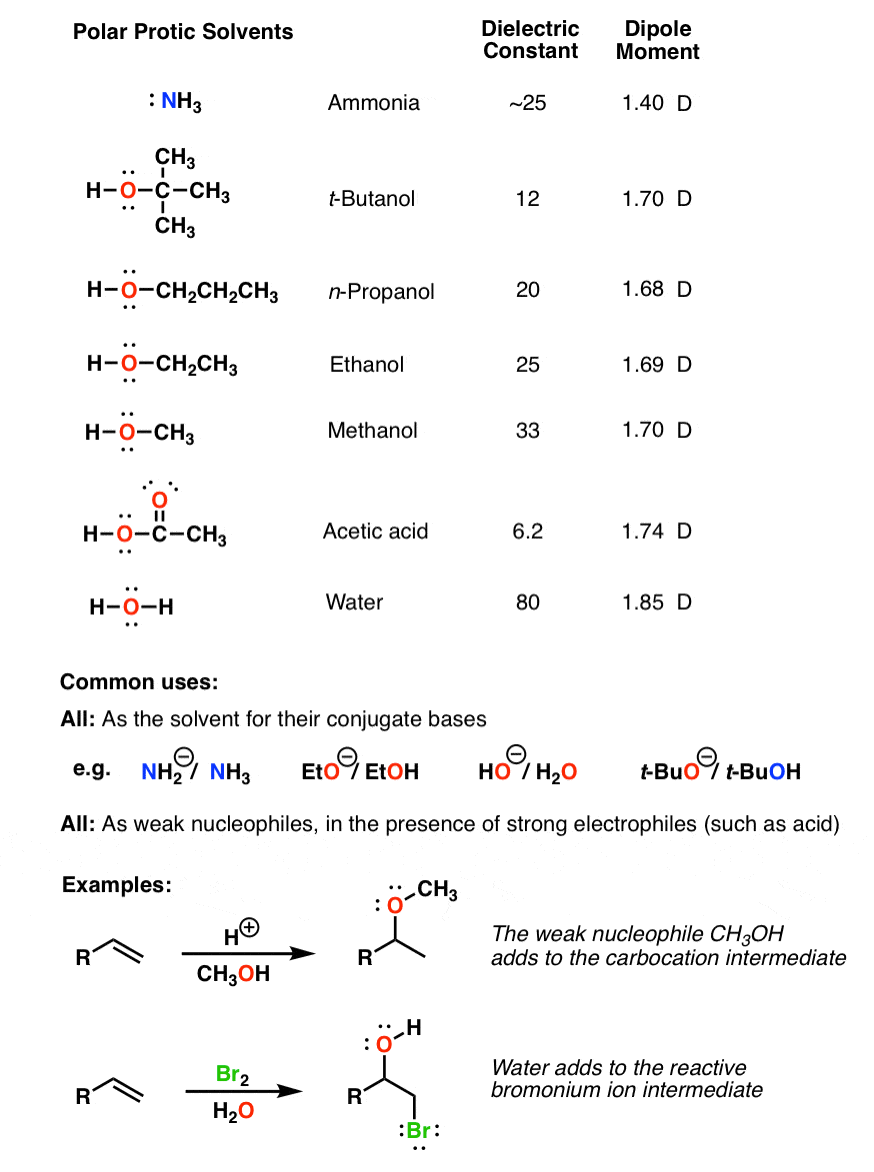
Polar Protic Polar Aprotic Nonpolar All About Solvents
Solutions. This further contributes the ability of hydrocarbon to become water insoluble. Polarity is quintessential when it comes to solubility: Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Study percent by mass, volume. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. This is where the term polar comes from: Water molecules have true electrical dipoles. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. However water and hexanes don't have anything in common. What makes hexane an interesting hydrocarbon compound is its insolubility in water. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. Explain why nacl dissolves in water. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). Hexane also does not conduct. Toluene is not a polar molecular it cannot dissolve in water which is polar.
Potentialities Of Using Liquefied Gases As Alternative Solvents To Substitute Hexane For The Extraction Of Aromas From Fresh And Dry Natural Products Sciencedirect
Chapter 4 Water Its Effect On Dissolved Biomolecules. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Toluene is not a polar molecular it cannot dissolve in water which is polar. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. What makes hexane an interesting hydrocarbon compound is its insolubility in water. However water and hexanes don't have anything in common. Polarity is quintessential when it comes to solubility: Hexane also does not conduct. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. This further contributes the ability of hydrocarbon to become water insoluble. Explain why nacl dissolves in water. Study percent by mass, volume. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). This is where the term polar comes from: Water molecules have true electrical dipoles. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the.
Ch104 Chapter 7 Solutions Chemistry
Chapter 1 Organic Chemistry Review Hydrocarbons Che 120 Introduction To Organic Chemistry Textbook Libguides At Hostos Community College Library. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). However water and hexanes don't have anything in common. Polarity is quintessential when it comes to solubility: What makes hexane an interesting hydrocarbon compound is its insolubility in water. Water molecules have true electrical dipoles. Hexane also does not conduct. Explain why nacl dissolves in water. This is where the term polar comes from: This further contributes the ability of hydrocarbon to become water insoluble. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Toluene is not a polar molecular it cannot dissolve in water which is polar. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. Study percent by mass, volume.
Solved Wear Safety Glasses While In The Lab Follow All O Chegg Com
10 1 Intermolecular Forces Chemistry. What makes hexane an interesting hydrocarbon compound is its insolubility in water. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). Polarity is quintessential when it comes to solubility: However water and hexanes don't have anything in common. Study percent by mass, volume. Hexane also does not conduct. Water molecules have true electrical dipoles. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Explain why nacl dissolves in water. Toluene is not a polar molecular it cannot dissolve in water which is polar. This is where the term polar comes from: (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. This further contributes the ability of hydrocarbon to become water insoluble.
Supplemental Topics
Chemistry Ii Water And Organic Molecules. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. Study percent by mass, volume. Polarity is quintessential when it comes to solubility: Water molecules have true electrical dipoles. This is where the term polar comes from: There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). Hexane also does not conduct. However water and hexanes don't have anything in common. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Explain why nacl dissolves in water. Toluene is not a polar molecular it cannot dissolve in water which is polar. What makes hexane an interesting hydrocarbon compound is its insolubility in water. This further contributes the ability of hydrocarbon to become water insoluble. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the.
Illustrated Glossary Of Organic Chemistry Polar Nonpolar
Solvation Wikipedia. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. What makes hexane an interesting hydrocarbon compound is its insolubility in water. However water and hexanes don't have anything in common. When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. This further contributes the ability of hydrocarbon to become water insoluble. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. Toluene is not a polar molecular it cannot dissolve in water which is polar. Polarity is quintessential when it comes to solubility: This is where the term polar comes from: Hexane also does not conduct. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. Explain why nacl dissolves in water. Study percent by mass, volume. Water molecules have true electrical dipoles.
How Is Molecular Polarity Related To Solubility Socratic
Solubility And Molecular Structure. There is not enough dipole movement to make it this will determine the polarity and this determines whther the salt will dsisolve in water ( a polar molecular). Study percent by mass, volume. Explain why nacl dissolves in water. However water and hexanes don't have anything in common. This further contributes the ability of hydrocarbon to become water insoluble. Water molecules have true electrical dipoles. Therefore, they are too different from each other and are not miscible.old (not so good) hexane is not soluble in water because hexane is a nonpolar solvent while water is polar solvent. (3) substances that are mostly nonpolar dissolve in nonpolar solvents like hexane but not in polar solvents like water, presumably since the. This is where the term polar comes from: Polar water molecules have a strong attraction for charged ions and the charged ions become solvated as they dissociate into water and ionic for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent and ionic compounds are insoluble in hexane. Toluene is not a polar molecular it cannot dissolve in water which is polar. Hexane also does not conduct. Polarity is quintessential when it comes to solubility: When water and hexane are mixed together, hexane is evidently seen to floating above the presence of water. What makes hexane an interesting hydrocarbon compound is its insolubility in water.