Does Hexane Soluble In Water. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. No hexane is insoluble in water. Hexane is one element that is symbolized by the chemical formula of c6h14. Hexane is a significant constituent of gasoline. Why is hexane not soluble in water? Polarity is quintessential when it comes to solubility: In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Such element has an alkaline property that consists of six carbon atoms. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. On the one hand, only compounds whose characteristics are similar (i.e.
Oneclass Part B Solubility Miscibility Of Different Alcohols Solvents Alcohols Methyl Alcohol Ch3
Solutions Like Dissolves Like Solubility And Intermolecular Forces Chemdemos. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Polarity is quintessential when it comes to solubility: Hexane is one element that is symbolized by the chemical formula of c6h14. Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Hexane is a significant constituent of gasoline. On the one hand, only compounds whose characteristics are similar (i.e. Why is hexane not soluble in water? Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Such element has an alkaline property that consists of six carbon atoms. No hexane is insoluble in water.
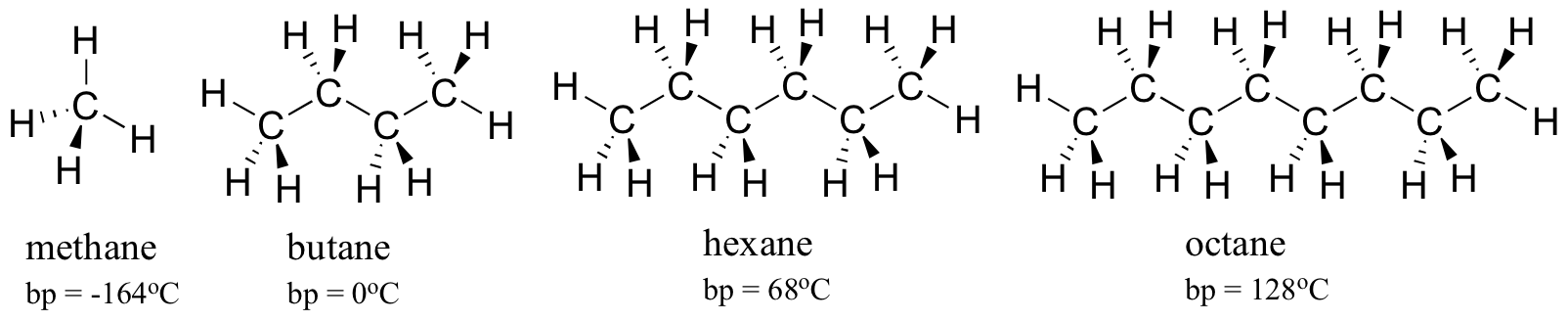
But i got it wrong.
This demonstrates that despite having a polar hydroxyl group that can form hydrogen bonds, alcohols become less soluble in water (and more soluble in nonpolar solvents) as the alkyl portion of the alcohol becomes larger. A wide variety of water soluble dyes options are available to you, such as ink dyestuffs, food dyestuffs, and textile. Do you call this soluble? Methyl alcohol was soluble in water and partially soluble in hexane. Because the solute is equally soluble in both, concentration in water = concentration in hexane. Popular soluble in water of good quality and at affordable prices you can buy on aliexpress. Water is a polar covalent substance and hexane is nonpolar. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. They do not dissolve in water. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Hexane is one element that is symbolized by the chemical formula of c6h14. Carboxylic acids are soluble in water because they can form hydrogen bonds with water. However, of the three choices. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. You have to consider similar things with diphenylamine and hcl and with phenol and naoh. 3 minutes ago the hec mberyllium = 10.1 g. At first , it is con fusing that what are you talking about. This demonstrates that despite having a polar hydroxyl group that can form hydrogen bonds, alcohols become less soluble in water (and more soluble in nonpolar solvents) as the alkyl portion of the alcohol becomes larger. Ionic compounds easily soluble in any liquid that is capable of breaking the ionic bond in them. After the oil has been extracted using an expeller, the remaining pulp can be further processed with (cyclo)hexane to extract the remaining oil content. Hexane solvent extraction can be used in isolation or along with the oil press/expeller method. Hexane is a significant constituent of gasoline. Such element has an alkaline property that consists of six carbon atoms. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Why is hexane not soluble in water? Polar solutes are more soluble in polar solvents (i.e., water), and nonpolar they do not exist in either hydrocarbon because only c−c, c=c, and c−h bonds exist, which do not have significant. Like butane, hexane is a simple alkane, but it has two more carbon atoms and four more hydrogen's, which makes it completely insoluble in water. Pentane, with five carbons in the chain, is the first completely insoluble alkane. Polar water molecules have a strong attraction for charged ions and the charged ions become solvated for instance, nonpolar molecular substances are likely to dissolve in hexane, which a nonpolar solvent. Polarity is quintessential when it comes to solubility: But i got it wrong.
Solubility Of Liquids
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gct5hc46xliyckmjqlryfqj4rm0qgfvs53braq Usqp Cau. Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. No hexane is insoluble in water. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Hexane is a significant constituent of gasoline. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. On the one hand, only compounds whose characteristics are similar (i.e. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Polarity is quintessential when it comes to solubility: Why is hexane not soluble in water? Such element has an alkaline property that consists of six carbon atoms. Hexane is one element that is symbolized by the chemical formula of c6h14. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon.
Chemistry The Central Science Chapter 13 Section 3
Why Does Hexane Dissolve In Acetone Quora. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. Hexane is a significant constituent of gasoline. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Why is hexane not soluble in water? Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. No hexane is insoluble in water. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Hexane is one element that is symbolized by the chemical formula of c6h14. Such element has an alkaline property that consists of six carbon atoms. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Polarity is quintessential when it comes to solubility: On the one hand, only compounds whose characteristics are similar (i.e. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres.
Solved Part A For Each Of The Following Molecules Would Chegg Com
Which Compound S Water Acetone Ethanol Hexane Decane 1 Decanol Sodium Chloride And Or Iodine Do You Think Will Homeworklib. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. Hexane is a significant constituent of gasoline. No hexane is insoluble in water. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Such element has an alkaline property that consists of six carbon atoms. Polarity is quintessential when it comes to solubility: Hexane is one element that is symbolized by the chemical formula of c6h14. Why is hexane not soluble in water? Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. On the one hand, only compounds whose characteristics are similar (i.e. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other.
Polar And Non Polar Solubility
Would You Expect Alanine An Amino Acid To Be Mo. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Hexane is one element that is symbolized by the chemical formula of c6h14. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. Polarity is quintessential when it comes to solubility: Such element has an alkaline property that consists of six carbon atoms. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. On the one hand, only compounds whose characteristics are similar (i.e. Why is hexane not soluble in water? Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Hexane is a significant constituent of gasoline. No hexane is insoluble in water.
Effects Of Etoh Extract Of A Tsaoko Seeds Water Soluble And Download Scientific Diagram
Comparing The Solubility Of Water Ethanol And Hexane Youtube. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. No hexane is insoluble in water. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Hexane is one element that is symbolized by the chemical formula of c6h14. On the one hand, only compounds whose characteristics are similar (i.e. Such element has an alkaline property that consists of six carbon atoms. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Hexane is a significant constituent of gasoline. Polarity is quintessential when it comes to solubility: Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. Why is hexane not soluble in water?
Determination Of Ph In Non Aqueous Solutions Horiba
Solved 1 For Each Compound Would You Expect Greater Sol Chegg Com. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. Such element has an alkaline property that consists of six carbon atoms. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. No hexane is insoluble in water. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. Hexane is a significant constituent of gasoline. On the one hand, only compounds whose characteristics are similar (i.e. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. Polarity is quintessential when it comes to solubility: Why is hexane not soluble in water? Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Hexane is one element that is symbolized by the chemical formula of c6h14.
Solved Questions 1 Would You Expect Calcium Chloride An Chegg Com
Why Is Hexane Insoluble In Water Quora. Polarity is quintessential when it comes to solubility: Polar vs nonpolar) mix well into a unknown liquid is insoluble in water and soluble in cyclohexane and alcohol with a boiling point of 57 c at 658 mm hg. On the one hand, only compounds whose characteristics are similar (i.e. Solubility of two chemical compounds (solvents) which merge to form one homogeneous blend is determined by the individual chemical properties of the constituent molecules of each solvent. Why is hexane not soluble in water? Such element has an alkaline property that consists of six carbon atoms. Hexane is a significant constituent of gasoline. Like dissolves like meaning the more alike (chemically) two things are the more likely they will be soluble in each other. Classified as a hydrocarbon, this element only contains the basic elements of hydrogen and carbon. Like dissolves like, halogens in organic solvent and water edexcel unit 1 and 2. Hexane is one element that is symbolized by the chemical formula of c6h14. .incorporate hexane in water as oil/water emulsion or you did mixing in the wrong way as vigorous shaking cause emulsion try to increase the hexane and shake gently in separator funnel how ever we extract flavonoid by aqueous ethanol not by water. In day to day chores we come across many examples where we mix two liquids or liquids with solids for. No hexane is insoluble in water. The vapour pressure of hexane above water containing just a few mg/l is as great as above pure hexane and can lead to explosive atmospheres.